Cancer Res Treat.
2023 Jan;55(1):83-93. 10.4143/crt.2021.1571.
Efficacy and Safety of Ceritinib 450 mg/day with Food and 750 mg/day in Fasted State in Treatment-Naïve Patients with ALK+ Non–Small Cell Lung Cancer: Results from the ASCEND-8 Asian Subgroup Analysis
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Medical Oncology, Rajiv Gandhi Cancer Institute and Research Center, New Delhi, India
- 4Division of Hematology-Oncology, Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 5Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 6Chang Gung Memorial Hospital and Chang Gung University, Taoyuan, Taiwan
- 7Hospital Umum Sarawak, Kuching, Malaysia
- 8Chulalongkorn University and The King Chulalongkorn Memorial Hospital, Bangkok, Thailand
- 9HCG Curie Center of Oncology and Kidwai Memorial Institute of Oncology, Bengaluru, India
- 10Novartis Pharma AG, Basel, Switzerland
- 11Novartis Pharmaceuticals Corporation, East Hanover, New Jersey, USA
- 12Songklanagarind Hospital, Prince of Songkla University, Songkhla, Thailand
- KMID: 2537994
- DOI: http://doi.org/10.4143/crt.2021.1571
Abstract
- Purpose
Previous report from the ASCEND-8 trial showed consistent efficacy with less gastrointestinal (GI) toxicity in patients with anaplastic lymphoma kinase-rearranged (ALK+) advanced/metastatic non–small cell lung cancer (NSCLC) treated with ceritinib 450-mg with food compared with 750-mg fasted. In this subgroup analysis, we report outcomes in Asian patients of the ASCEND-8 trial.
Materials and Methods
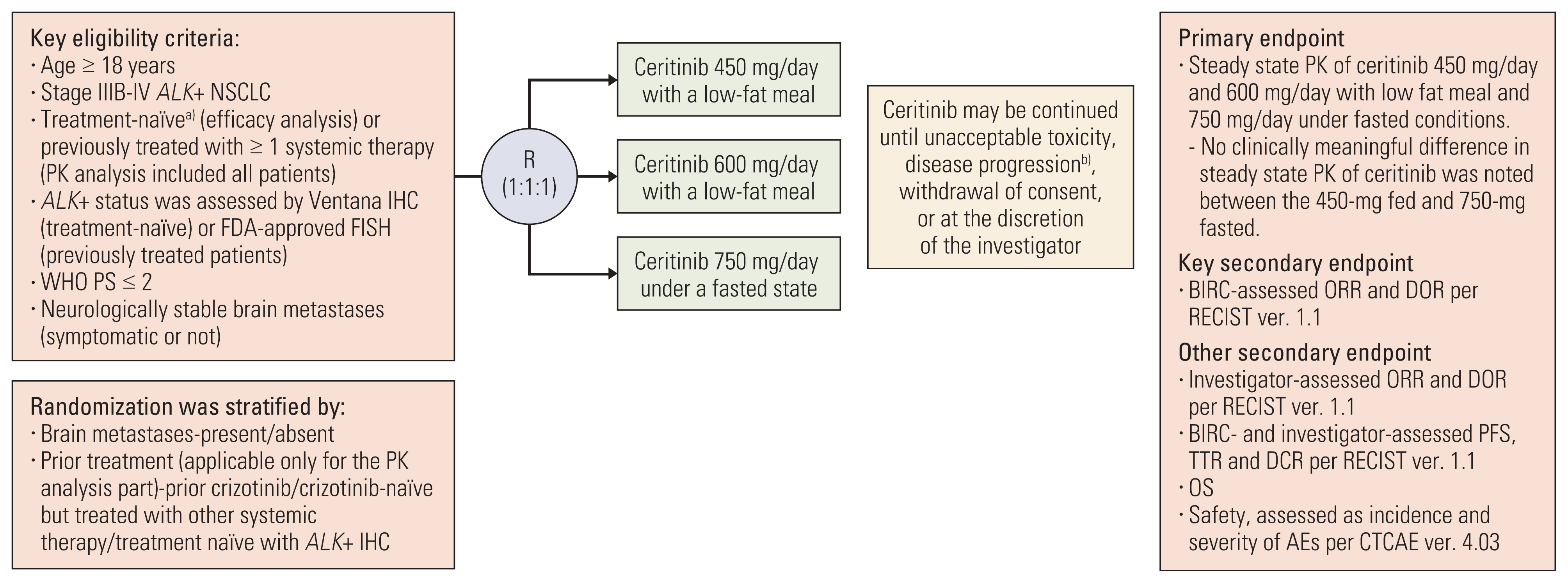
Key efficacy endpoints were blinded independent review committee (BIRC)–assessed overall response rate (ORR) and duration of response (DOR) evaluated per Response Evaluation Criteria in Solid Tumors v1.1. Other efficacy endpoints were investigator-assessed ORR and DOR; BIRC- and investigator-assessed progression-free survival (PFS) and disease control rate; overall survival (OS). Safety was evaluated by frequency and severity of adverse events.
Results
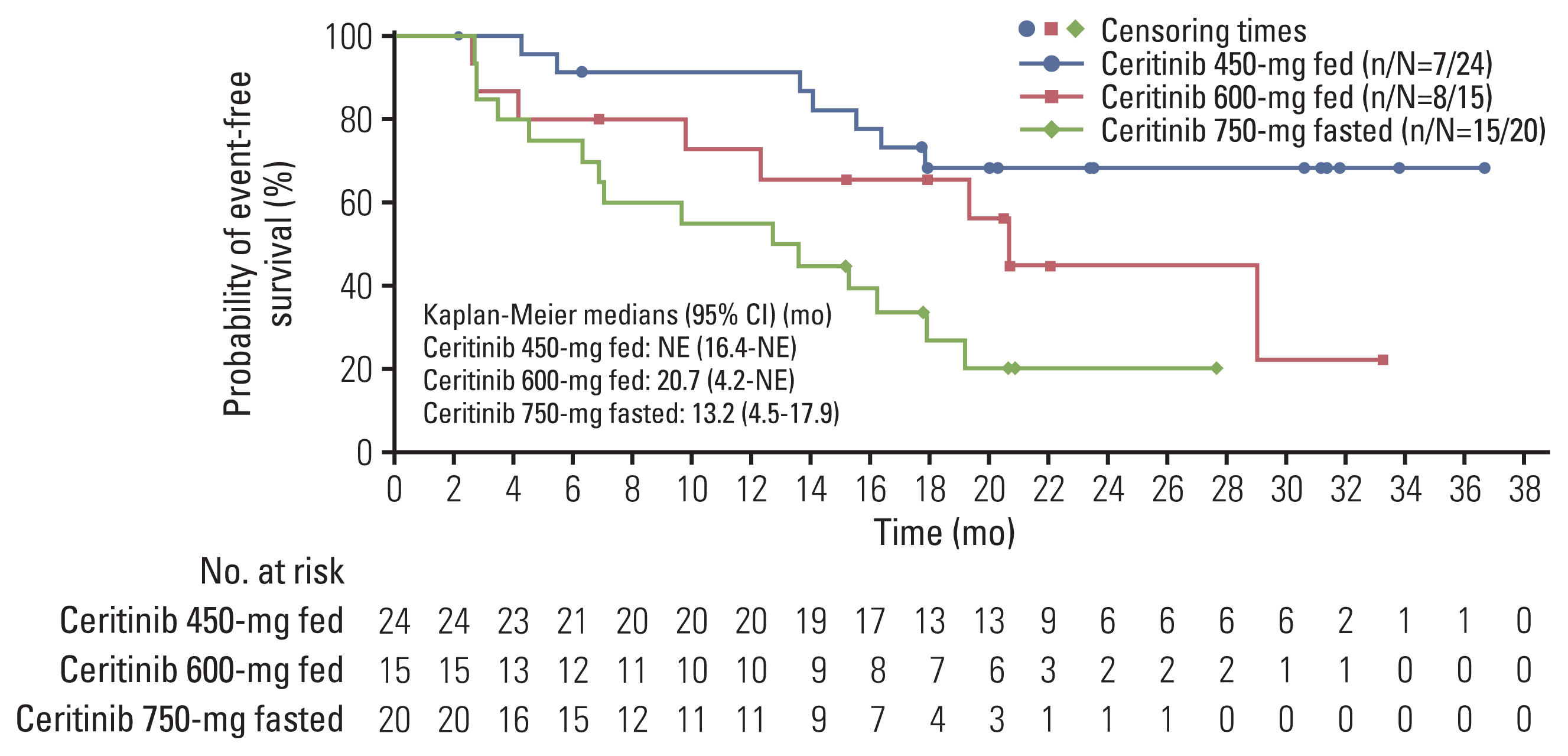
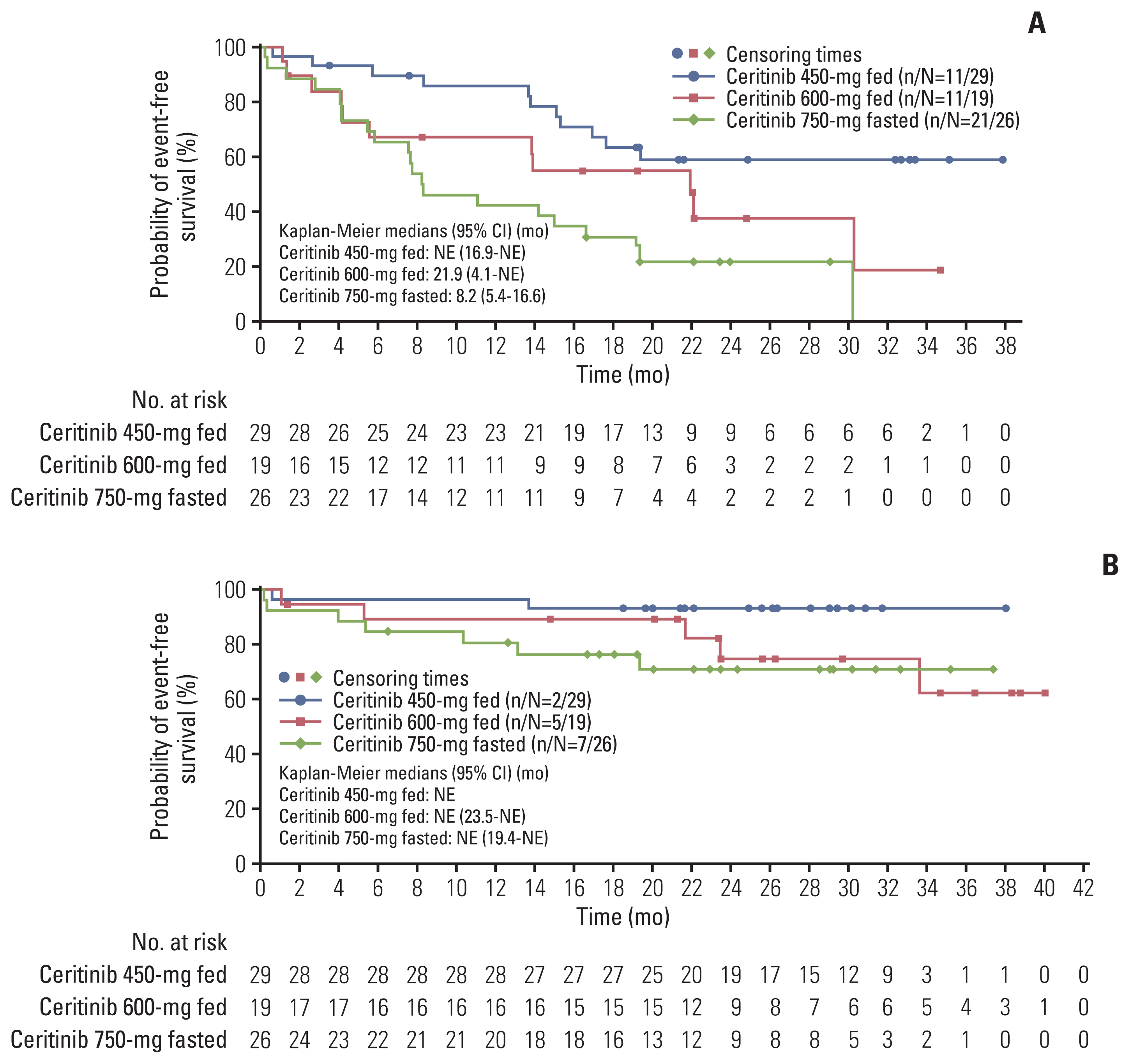
At final data cutoff (6 March 2020), 198 treatment-naïve patients were included in efficacy analysis, of which 74 (37%) comprised the Asian subset; 450-mg fed (n=29), 600-mg fed (n=19), and 750-mg fasted (n=26). Baseline characteristics were mostly comparable across study arms. At baseline, more patients in 450-mg fed arm (44.8%) had brain metastases than in 750-mg fasted arm (26.9%). Per BIRC, patients in the 450-mg fed arm had a numerically higher ORR, 24-month DOR rate and 24-month PFS rate than the 750-mg fasted arm. The 36-month OS rate was 93.1% in 450-mg fed arm and 70.9% in 750-mg fasted arm. Any-grade GI toxicity occurred in 82.8% and 96.2% of patients in the 450-mg fed and 750-mg fasted arms, respectively.
Conclusion
Asian patients with ALK+ advanced/metastatic NSCLC treated with ceritinib 450-mg fed showed numerically higher efficacy and lower GI toxicity than 750-mg fasted patients.
Figure
Reference
-
References
1. Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007; 448:561–6.
Article2. Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014; 371:2167–77.
Article3. Soria JC, Tan DS, Chiari R, Wu YL, Paz-Ares L, Wolf J, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017; 389:917–29.4. U.S. Food and Drug Administration. Xalkori prescribing information [Internet]. Silver Spring MD: U.S. Food and Drug Administration;2017. [cited 2021 Oct 15]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202570s021lbl.pdf .5. U.S. Food and Drug Administration. Zykadia prescribing information [Internet]. Silver Spring MD: U.S. Food and Drug Administration;2019. [cited 2021 Oct 15]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211225s000lbl.pdf .6. Gristina V, La Mantia M, Iacono F, Galvano A, Russo A, Bazan V. The emerging therapeutic landscape of ALK inhibitors in non-small cell lung cancer. Pharmaceuticals (Basel). 2020; 13:474.
Article7. U.S. Food and Drug Administration. FDA broadens ceritinib indication to previously untreated ALK-positive metastatic NSCLC [Internet]. Spring, MD: U.S. Food and Drug Administration;c2017. [cited 2021 May 8]. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-broadens-ceritinib-indication-previously-untreated-alk-positive-metastatic-NSCLC .8. Shaw AT, Kim TM, Crino L, Gridelli C, Kiura K, Liu G, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2017; 18:874–86.
Article9. Crino L, Ahn MJ, De Marinis F, Groen HJ, Wakelee H, Hida T, et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J Clin Oncol. 2016; 34:2866–73.10. Kim DW, Mehra R, Tan DSW, Felip E, Chow LQ, Camidge DR, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016; 17:452–63.
Article11. Cho BC, Kim DW, Bearz A, Laurie SA, McKeage M, Borra G, et al. ASCEND-8: a randomized phase 1 study of ceritinib, 450 mg or 600 mg, taken with a low-fat meal versus 750 mg in fasted state in patients with snaplastic lymphoma kinase (ALK)-rearranged metastatic non-small cell lung cancer (NSCLC). J Thorac Oncol. 2017; 12:1357–67.12. Cho BC, Obermannova R, Bearz A, McKeage M, Kim DW, Batra U, et al. Efficacy and safety of ceritinib (450 mg/d or 600 mg/d) with food versus 750-mg/d fasted in patients with ALK receptor tyrosine kinase (ALK)-positive NSCLC: primary efficacy results from the ASCEND-8 study. J Thorac Oncol. 2019; 14:1255–65.
Article13. Tan DS, Geater S, Yu CJ, Tsai CM, Hsia TC, Chen J, et al. Ceritinib efficacy and safety in treatment-naive Asian patients with advanced ALK-rearranged NSCLC: an ASCEND-4 subgroup analysis. JTO Clin Res Rep. 2021; 2:100131.
Article14. Zhou C, Kim SW, Reungwetwattana T, Zhou J, Zhang Y, He J, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. 2019; 7:437–46.
Article15. Mok TS, Peters S, Camidge DR, Ou SH, Ahn JS, Tan EH, et al. Alectinib (ALC) vs crizotinib (CRZ) in treatment-naïve ALK+ non-small-cell lung cancer (NSCLC): Asian vs non-Asian subgroup analysis of the ALEX study. Ann Oncol. 2017; 28(Suppl 10):X191.
Article16. Nishio M, Kim DW, Wu YL, Nakagawa K, Solomon BJ, Shaw AT, et al. Crizotinib versus chemotherapy in Asian patients with ALK-positive advanced non-small cell lung cancer. Cancer Res Treat. 2018; 50:691–700.
Article17. Wu YL, Lu S, Lu Y, Zhou J, Shi YK, Sriuranpong V, et al. Results of PROFILE 1029, a phase III comparison of first-line crizotinib versus chemotherapy in East Asian patients with ALK-positive advanced non-small cell lung cancer. J Thorac Oncol. 2018; 13:1539–48.18. Mok T, Camidge DR, Gadgeel SM, Rosell R, Dziadziuszko R, Kim DW, et al. Updated overall survival and final progression-free survival data for patients with treatment-naive advanced ALK-positive non-small-cell lung cancer in the ALEX study. Ann Oncol. 2020; 31:1056–64.19. Li H, Lai L, Wu B. Cost effectiveness of ceritinib and alectinib versus crizotinib in first-line anaplastic lymphoma kinase-positive advanced non-small-cell lung cancer. Clin Drug Investig. 2020; 40:183–9.
Article20. Gainor JF, Ou SH, Logan J, Borges LF, Shaw AT. The central nervous system as a sanctuary site in ALK-positive non-small-cell lung cancer. J Thorac Oncol. 2013; 8:1570–3.
Article21. Ali A, Goffin JR, Arnold A, Ellis PM. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol. 2013; 20:e300–6.
Article22. Gaspar LE, Chansky K, Albain KS, Vallieres E, Rusch V, Crowley JJ, et al. Time from treatment to subsequent diagnosis of brain metastases in stage III non-small-cell lung cancer: a retrospective review by the Southwest Oncology Group. J Clin Oncol. 2005; 23:2955–61.23. Lee SJ, Lee JI, Nam DH, Ahn YC, Han JH, Sun JM, et al. Leptomeningeal carcinomatosis in non-small-cell lung cancer patients: impact on survival and correlated prognostic factors. J Thorac Oncol. 2013; 8:185–91.24. Sperduto PW, Kased N, Roberge D, Xu Z, Shanley R, Luo X, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012; 30:419–25.
Article25. Chow LQ, Barlesi F, Bertino EM, van den Bent MJ, Wakelee H, Wen PY, et al. Results of the ASCEND-7 phase II study evaluating ALK inhibitor (ALKi) ceritinib in patients (pts) with ALK+ non-small cell lung cancer (NSCLC) metastatic to the brain. Ann Oncol. 2019; 30(Suppl 5):v602–60.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Phase II Study of Vinorelbine, Mitomycin C and Cisplatin Chemotherapy for Advanced Non-Small Cell Lung Cancer
- Crizotinib versus Chemotherapy in Asian Patients with ALK-Positive Advanced Non-small Cell Lung Cancer
- Etoposide and cisplatin combination chemotherapy in extensive stage small cell lung cancer
- The Effect of Combination Chemotherapy with Vinorelbine, Carboplatin, and Ifosfamide in Patients with Advanced Non-Small Cell Lung Cancer
- Bilateral Ovarian Metastases from ALK Rearranged Non-Small Cell Lung Cancer