J Korean Med Sci.
2022 Dec;37(48):e338. 10.3346/jkms.2022.37.e338.
Increased Pro-Inflammatory T Cells, Senescent T Cells, and Immune-Check Point Molecules in the Placentas of Patients With Gestational Diabetes Mellitus
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Chungnam National University College of Medicine, Daejeon, Korea
- 2Laboratory of Endocrinology and Immune System, Chungnam National University College of Medicine, Daejeon, Korea
- 3Department of Pathology, Chungnam National University College of Medicine, Daejeon, Korea
- 4Department of Obstetrics and Gynecology, Chungnam National University Hospital, Daejeon, Korea
- 5Department of Obstetrics and Gynecology, Chungnam National University Sejong Hospital, Sejong, Korea
- 6Department of Obstetrics and Gynecology, Chungnam National University College of Medicine, Daejeon, Korea
- KMID: 2536732
- DOI: http://doi.org/10.3346/jkms.2022.37.e338
Abstract
- Background
Gestational diabetes mellitus (GDM) is the most common metabolic complication of pregnancy. To define the altered pathway in GDM placenta, we investigated the transcriptomic profiles from human placenta between GDM and controls.
Methods
Clinical parameters and postpartum complications were reviewed in all participants. Differentially expressed canonical pathways were analyzed between the GDM and control groups based on transcriptomic analysis. CD4 + T, CD8 + T, and senescent T cell subsets were determined by flow cytometry based on staining for specific intracellular cytokines.
Results
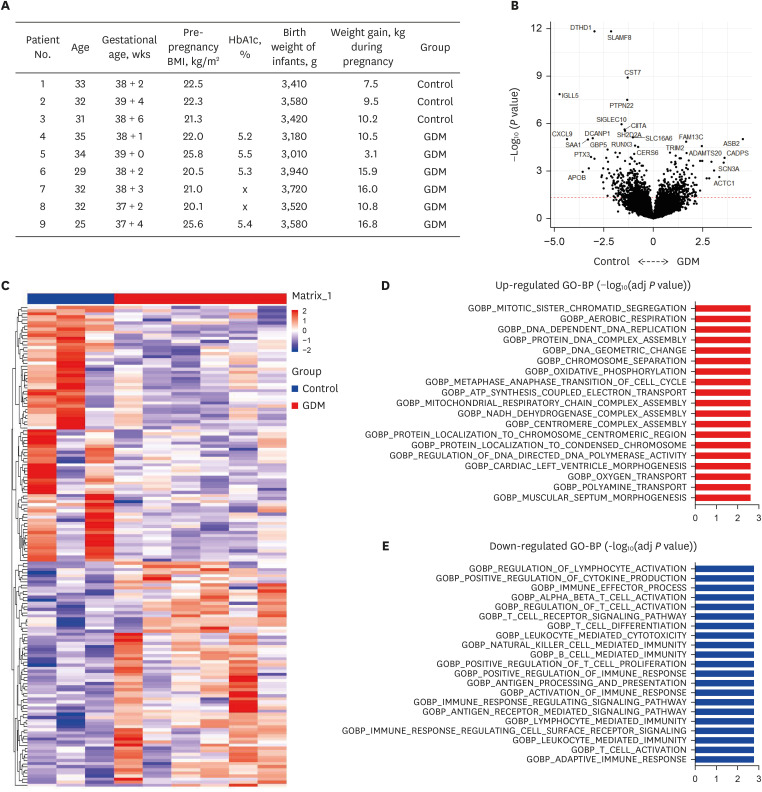
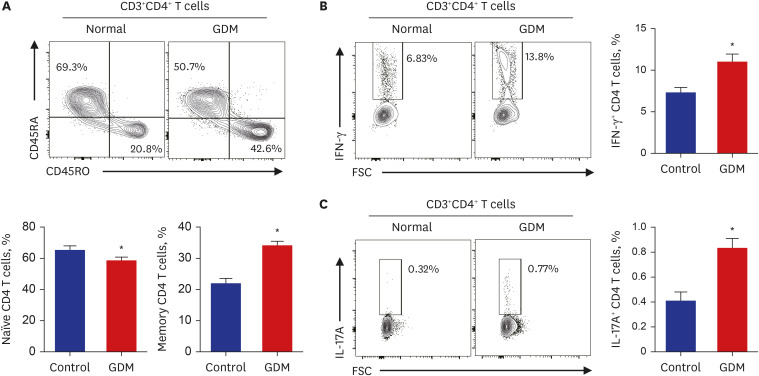
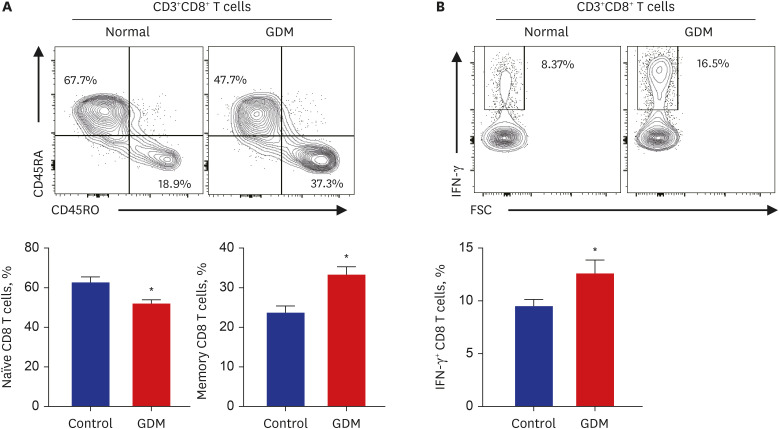
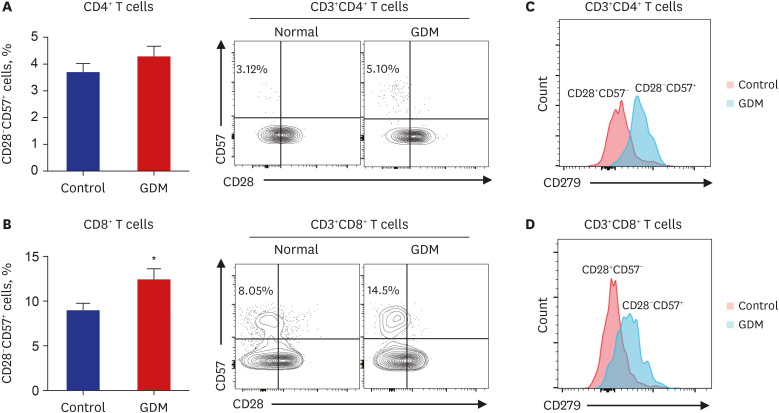
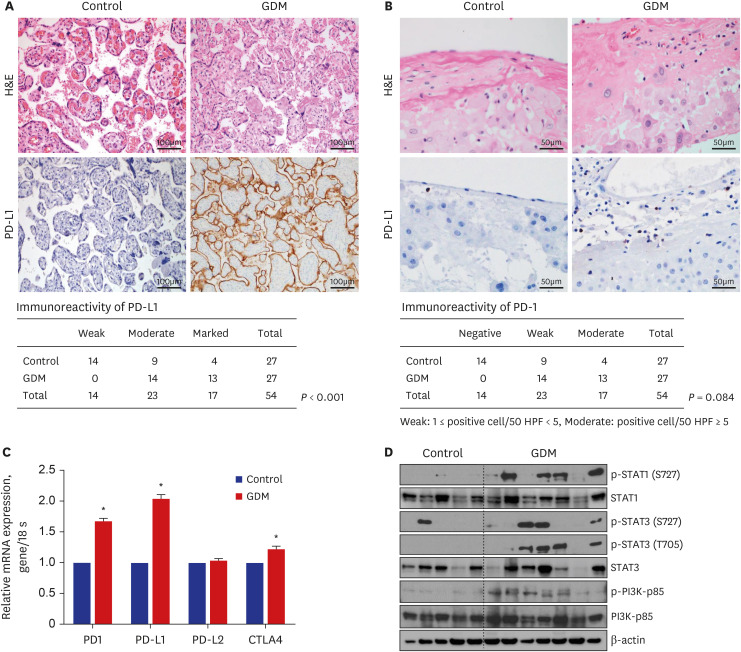
Gene ontology analysis revealed that the placenta of GDM revealed upregulation of diverse mitochondria or DNA replication related pathways and downregulation of T-cell immunity related pathways. The maternal placenta of the GDM group had a higher proportion of CD4 + T and CD8 + T cells than the control group. Interestingly, senescent CD4 + T cells tended to increase and CD8 + T cells were significantly increased in GDM compared to controls, along with increased programmed cell death-1 (CD274 + ) expression. Programmed death-ligand 1 expression in syncytotrophoblasts was also significantly increased in patients with GDM.
Conclusion
This study demonstrated increased proinflammatory T cells, senescent T cells and immune-check point molecules in GDM placentas, suggesting that changes in senescent T cells and immune-escape signaling might be related to the pathophysiology of GDM.
Keyword
Figure
Cited by 1 articles
-
T-Cell Senescence in Human Metabolic Diseases
Ha Thi Nga, Thi Linh Nguyen, Hyon-Seung Yi
Diabetes Metab J. 2024;48(5):864-881. doi: 10.4093/dmj.2024.0140.
Reference
-
1. Bener A, Saleh NM, Al-Hamaq A. Prevalence of gestational diabetes and associated maternal and neonatal complications in a fast-developing community: global comparisons. Int J Womens Health. 2011; 3:367–373. PMID: 22140323.2. Cho KH, Yoon SJ, Lim J, Eun H, Park MS, Park KI, et al. Epidemiology of macrosomia in Korea: growth and development. J Korean Med Sci. 2021; 36(47):e320. PMID: 34873886.3. Chung HR, Moon JH, Lim JS, Lee YA, Shin CH, Hong JS, et al. Maternal hyperglycemia during pregnancy increases adiposity of offspring. Diabetes Metab J. 2021; 45(5):730–738. PMID: 33618504.4. Yu SH, Cho B, Lee Y, Kim E, Choi SH, Lim S, et al. Insulin secretion and incretin hormone concentration in women with previous gestational diabetes mellitus. Diabetes Metab J. 2011; 35(1):58–64. PMID: 21537414.5. Jang HC. Gestational diabetes in Korea: incidence and risk factors of diabetes in women with previous gestational diabetes. Diabetes Metab J. 2011; 35(1):1–7. PMID: 21537406.6. Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The pathophysiology of gestational diabetes mellitus. Int J Mol Sci. 2018; 19(11):3342. PMID: 30373146.7. Johns EC, Denison FC, Norman JE, Reynolds RM. Gestational diabetes mellitus: mechanisms, treatment, and complications. Trends Endocrinol Metab. 2018; 29(11):743–754. PMID: 30297319.8. Arck PC, Hecher K. Fetomaternal immune cross-talk and its consequences for maternal and offspring’s health. Nat Med. 2013; 19(5):548–556. PMID: 23652115.9. Alijotas-Reig J, Llurba E, Gris JM. Potentiating maternal immune tolerance in pregnancy: a new challenging role for regulatory T cells. Placenta. 2014; 35(4):241–248. PMID: 24581729.10. Fu B, Tian Z, Wei H. TH17 cells in human recurrent pregnancy loss and pre-eclampsia. Cell Mol Immunol. 2014; 11(6):564–570. PMID: 25027967.11. Yang H, Qiu L, Chen G, Ye Z, Lü C, Lin Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril. 2008; 89(3):656–661. PMID: 17543960.12. Santner-Nanan B, Peek MJ, Khanam R, Richarts L, Zhu E, Fazekas de St Groth B, et al. Systemic increase in the ratio between Foxp3+ and IL-17-producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol. 2009; 183(11):7023–7030. PMID: 19915051.13. Toldi G, Vásárhelyi ZE, Rigó J Jr, Orbán C, Tamássy Z, Bajnok A, et al. Prevalence of regulatory T-cell subtypes in preeclampsia. Am J Reprod Immunol. 2015; 74(2):110–115. PMID: 25816701.14. Vrachnis N, Belitsos P, Sifakis S, Dafopoulos K, Siristatidis C, Pappa KI, et al. Role of adipokines and other inflammatory mediators in gestational diabetes mellitus and previous gestational diabetes mellitus. Int J Endocrinol. 2012; 2012:549748. PMID: 22550485.15. Yang Y, Liu L, Liu B, Li Q, Wang Z, Fan S, et al. Functional defects of regulatory T cell through interleukin 10 mediated mechanism in the induction of gestational diabetes mellitus. DNA Cell Biol. 2018; 37(3):278–285. PMID: 29298097.16. Lapolla A, Dalfrà MG, Sanzari M, Fedele D, Betterle C, Masin M, et al. Lymphocyte subsets and cytokines in women with gestational diabetes mellitus and their newborn. Cytokine. 2005; 31(4):280–287. PMID: 15979891.17. Pendeloski KP, Mattar R, Torloni MR, Gomes CP, Alexandre SM, Daher S. Immunoregulatory molecules in patients with gestational diabetes mellitus. Endocrine. 2015; 50(1):99–109. PMID: 25754913.18. Yang Y, Guo F, Peng Y, Chen R, Zhou W, Wang H, et al. Transcriptomic profiling of human placenta in gestational diabetes mellitus at the single-cell level. Front Endocrinol (Lausanne). 2021; 12:679582. PMID: 34025588.19. Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD, et al. Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci U S A. 2015; 112(21):6682–6687. PMID: 25964334.20. Zamani MR, Aslani S, Salmaninejad A, Javan MR, Rezaei N. PD-1/PD-L and autoimmunity: a growing relationship. Cell Immunol. 2016; 310:27–41. PMID: 27660198.21. Zhang YH, Tian M, Tang MX, Liu ZZ, Liao AH. Recent insight into the role of the PD-1/PD-L1 pathway in feto-maternal tolerance and pregnancy. Am J Reprod Immunol. 2015; 74(3):201–208. PMID: 25640631.22. Fujisawa R, Haseda F, Tsutsumi C, Hiromine Y, Noso S, Kawabata Y, et al. Low programmed cell death-1 (PD-1) expression in peripheral CD4+ T cells in Japanese patients with autoimmune type 1 diabetes. Clin Exp Immunol. 2015; 180(3):452–457. PMID: 25682896.23. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabetes Care. 2021; 44(Suppl 1):S15–S33. PMID: 33298413.24. Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012; 7(3):562–578. PMID: 22383036.25. Yi HS, Kim SY, Kim JT, Lee YS, Moon JS, Kim M, et al. T-cell senescence contributes to abnormal glucose homeostasis in humans and mice. Cell Death Dis. 2019; 10(3):249. PMID: 30867412.26. Lee M, Hwang JT, Lee HJ, Jung SN, Kang I, Chi SG, et al. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. J Biol Chem. 2003; 278(41):39653–39661. PMID: 12900407.27. Jung SN, Shin DS, Kim HN, Jeon YJ, Yun J, Lee YJ, et al. Sugiol inhibits STAT3 activity via regulation of transketolase and ROS-mediated ERK activation in DU145 prostate carcinoma cells. Biochem Pharmacol. 2015; 97(1):38–50. PMID: 26212545.28. Pearce EL. Metabolism in T cell activation and differentiation. Curr Opin Immunol. 2010; 22(3):314–320. PMID: 20189791.29. Sheu A, Chan Y, Ferguson A, Bakhtyari MB, Hawke W, White C, et al. A proinflammatory CD4+ T cell phenotype in gestational diabetes mellitus. Diabetologia. 2018; 61(7):1633–1643. PMID: 29691600.30. Magee TR, Ross MG, Wedekind L, Desai M, Kjos S, Belkacemi L. Gestational diabetes mellitus alters apoptotic and inflammatory gene expression of trophobasts from human term placenta. J Diabetes Complications. 2014; 28(4):448–459. PMID: 24768206.31. Gomes CP, Torloni MR, Gueuvoghlanian-Silva BY, Alexandre SM, Mattar R, Daher S. Cytokine levels in gestational diabetes mellitus: a systematic review of the literature. Am J Reprod Immunol. 2013; 69(6):545–557. PMID: 23414425.32. Weng NP, Akbar AN, Goronzy J. CD28- T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009; 30(7):306–312. PMID: 19540809.33. Palmer BE, Blyveis N, Fontenot AP, Wilson CC. Functional and phenotypic characterization of CD57+CD4+ T cells and their association with HIV-1-induced T cell dysfunction. J Immunol. 2005; 175(12):8415–8423. PMID: 16339584.34. Covre LP, De Maeyer RP, Gomes DC, Akbar AN. The role of senescent T cells in immunopathology. Aging Cell. 2020; 19(12):e13272. PMID: 33166035.35. Miko E, Meggyes M, Doba K, Barakonyi A, Szereday L. Immune checkpoint molecules in reproductive immunology. Front Immunol. 2019; 10:846. PMID: 31057559.36. Tripathi S, Guleria I. Role of PD1/PDL1 pathway, and TH17 and Treg cells in maternal tolerance to the fetus. Biomed J. 2015; 38(1):25–31. PMID: 25355392.37. LaMarca B, Cornelius DC, Harmon AC, Amaral LM, Cunningham MW, Faulkner JL, et al. Identifying immune mechanisms mediating the hypertension during preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2016; 311(1):R1–R9. PMID: 27097659.38. Guleria I, Khosroshahi A, Ansari MJ, Habicht A, Azuma M, Yagita H, et al. A critical role for the programmed death ligand 1 in fetomaternal tolerance. J Exp Med. 2005; 202(2):231–237. PMID: 16027236.39. Enninga EA, Harrington SM, Creedon DJ, Ruano R, Markovic SN, Dong H, et al. Immune checkpoint molecules soluble program death ligand 1 and galectin-9 are increased in pregnancy. Am J Reprod Immunol. 2018; 79(2):e12795. PMID: 29205636.40. Veras E, Kurman RJ, Wang TL, Shih IM. PD-L1 expression in human placentas and gestational trophoblastic diseases. Int J Gynecol Pathol. 2017; 36(2):146–153. PMID: 27362903.41. Pillay P, Maharaj N, Moodley J, Mackraj I. Placental exosomes and pre-eclampsia: maternal circulating levels in normal pregnancies and, early and late onset pre-eclamptic pregnancies. Placenta. 2016; 46:18–25. PMID: 27697217.42. Li G, Lu C, Gao J, Wang X, Wu H, Lee C, et al. Association between PD-1/PD-L1 and T regulate cells in early recurrent miscarriage. Int J Clin Exp Pathol. 2015; 8(6):6512–6518. PMID: 26261529.43. Ye X, Ju S, Duan H, Yao Y, Wu J, Zhong S, et al. Immune checkpoint molecule PD-1 acts as a novel biomarker for the pathological process of gestational diabetes mellitus. Biomarkers Med. 2017; 11(9):741–749.44. Handwerger S, Freemark M. The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J Pediatr Endocrinol Metab. 2000; 13(4):343–356. PMID: 10776988.45. Grissa O, Yessoufou A, Mrisak I, Hichami A, Amoussou-Guenou D, Grissa A, et al. Growth factor concentrations and their placental mRNA expression are modulated in gestational diabetes mellitus: possible interactions with macrosomia. BMC Pregnancy Childbirth. 2010; 10(1):7. PMID: 20144210.46. Jarmuzek P, Wielgos M, Bomba-Opon D. Placental pathologic changes in gestational diabetes mellitus. Neuroendocrinol Lett. 2015; 36(2):101–105. PMID: 26071574.47. Olmos-Ortiz A, Flores-Espinosa P, Díaz L, Velázquez P, Ramírez-Isarraraz C, Zaga-Clavellina V. Immunoendocrine dysregulation during gestational diabetes mellitus: the central role of the placenta. Int J Mol Sci. 2021; 22(15):8087. PMID: 34360849.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regulation of Cellular Senescence in Type 2 Diabetes Mellitus: From Mechanisms to Clinical Applications
- T-Cell Senescence in Human Metabolic Diseases
- The Senolytic Drug JQ1 Removes Senescent Cells via Ferroptosis
- Recent advances in gestational diabetes mellitus
- IL-17A-Producing Foxp3⺠Regulatory T Cells and Human Diseases