Int J Stem Cells.
2022 Nov;15(4):372-383. 10.15283/ijsc22024.
LIPUS Promotes Endothelial Differentiation and Angiogenesis of Periodontal Ligament Stem Cells by Activating Piezo1
- Affiliations
-
- 1Department of Preventive Dentistry, College of Stomatology, Chongqing Medical University, Chongqing, China
- 2Chongqing Key Laboratory of Oral Diseases and Biomedical Sciences, Chongqing, China
- 3Chongqing Municipal Key Laboratory of Oral Biomedical Engineering of Higher Education, Chongqing, China
- KMID: 2536207
- DOI: http://doi.org/10.15283/ijsc22024
Abstract
- Background and Objectives
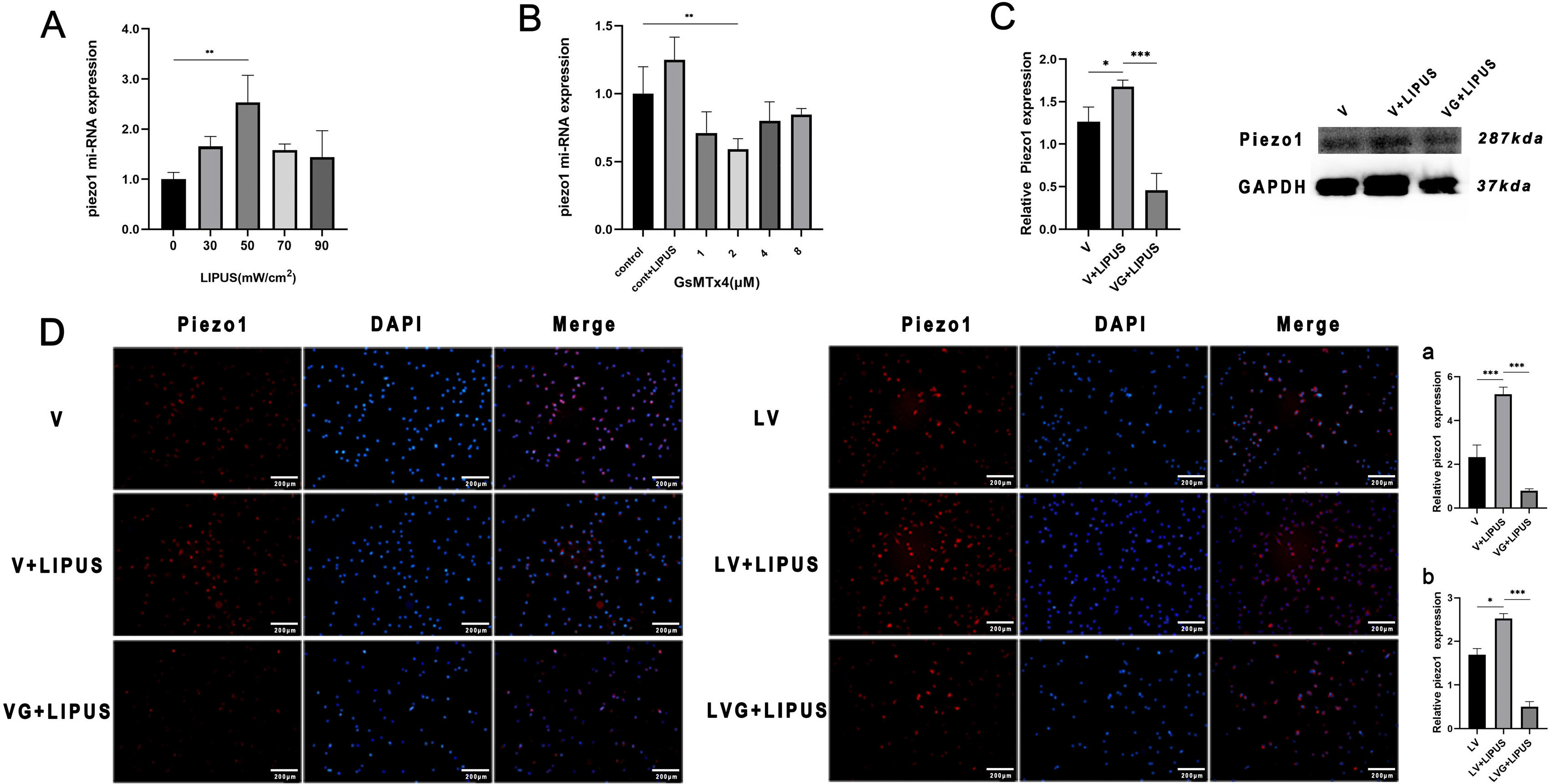
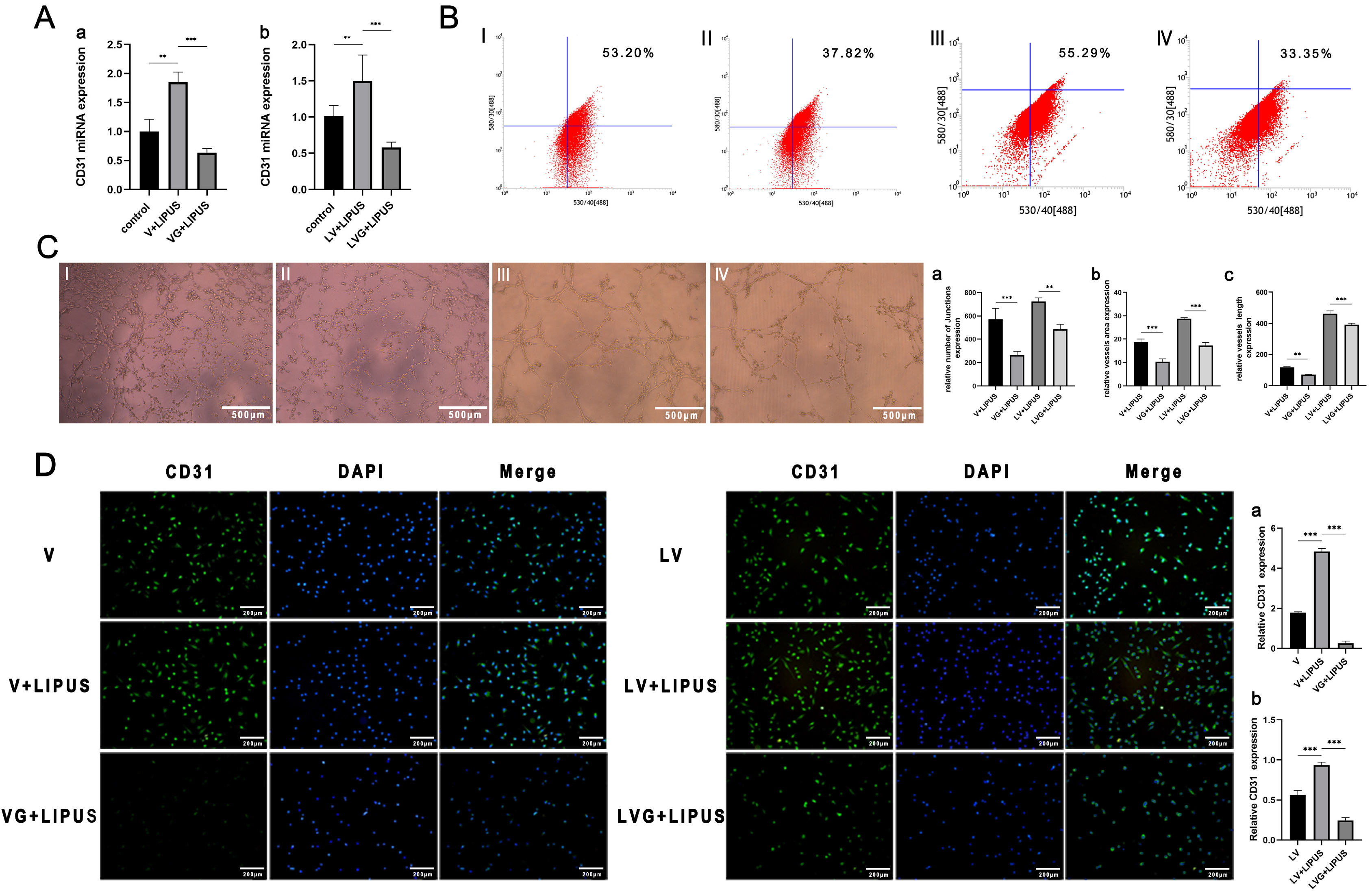
Low-intensity pulsed ultrasound (LIPUS) promotes differentiation and regulates biological functions of various stem cells, but its effect on the endothelial differentiation of periodontal ligament stem cells (PDLSCs) is unclear. This study investigated the effect of LIPUS on endothelial differentiation and angiogenesis in PDLSCs and the role of the mechanically sensitive ion channel Piezo1 in this process.
Methods and Results
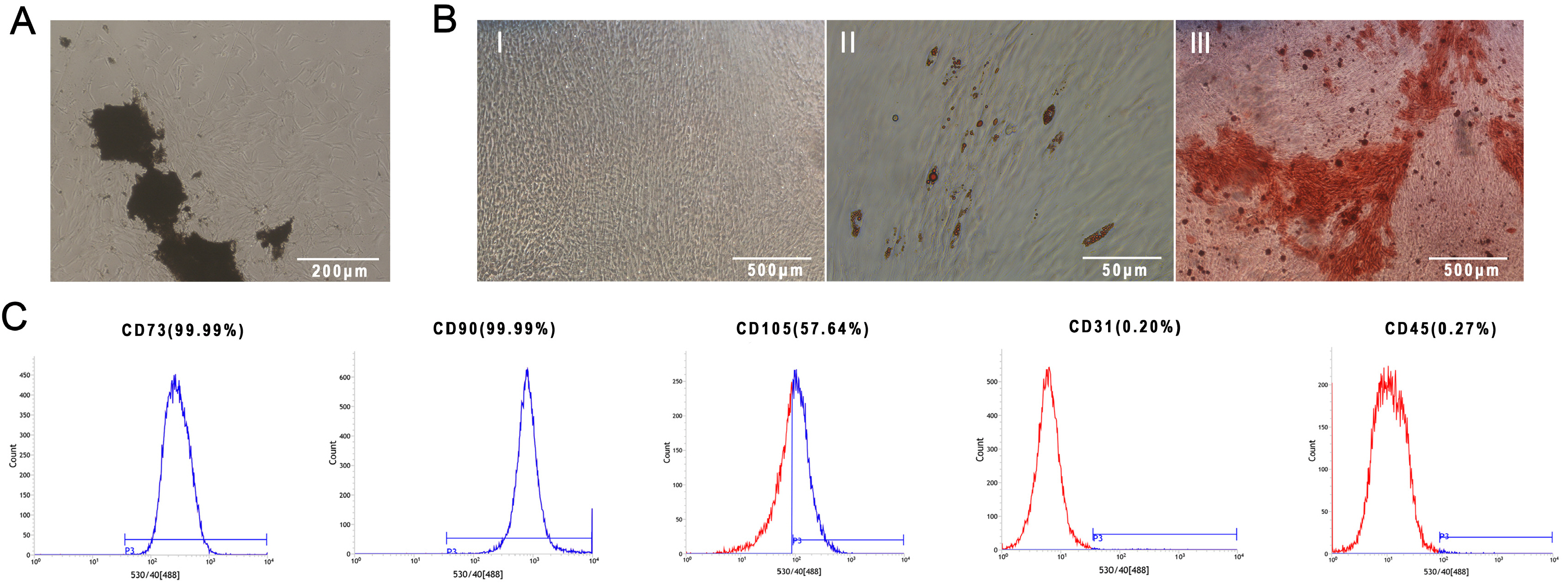
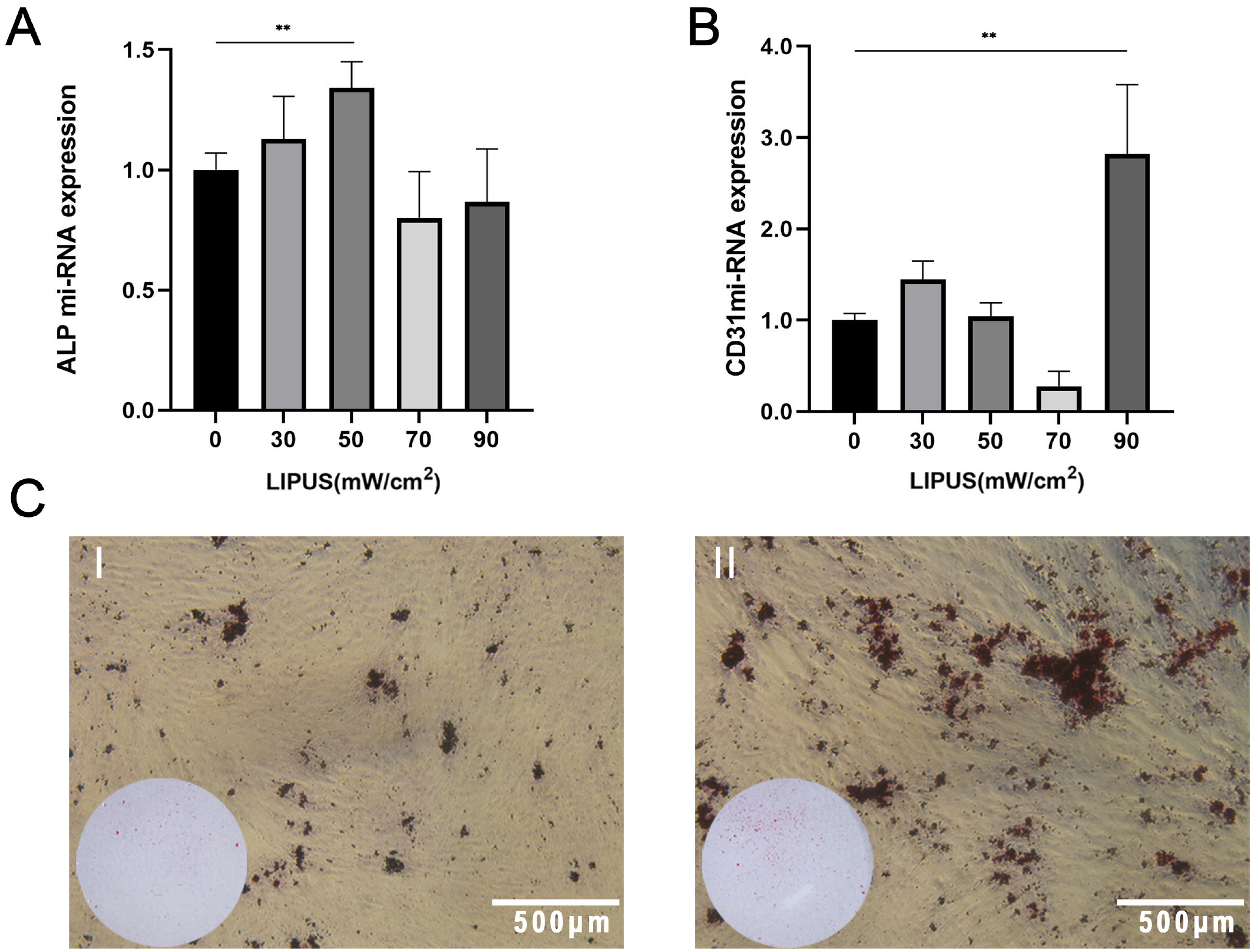
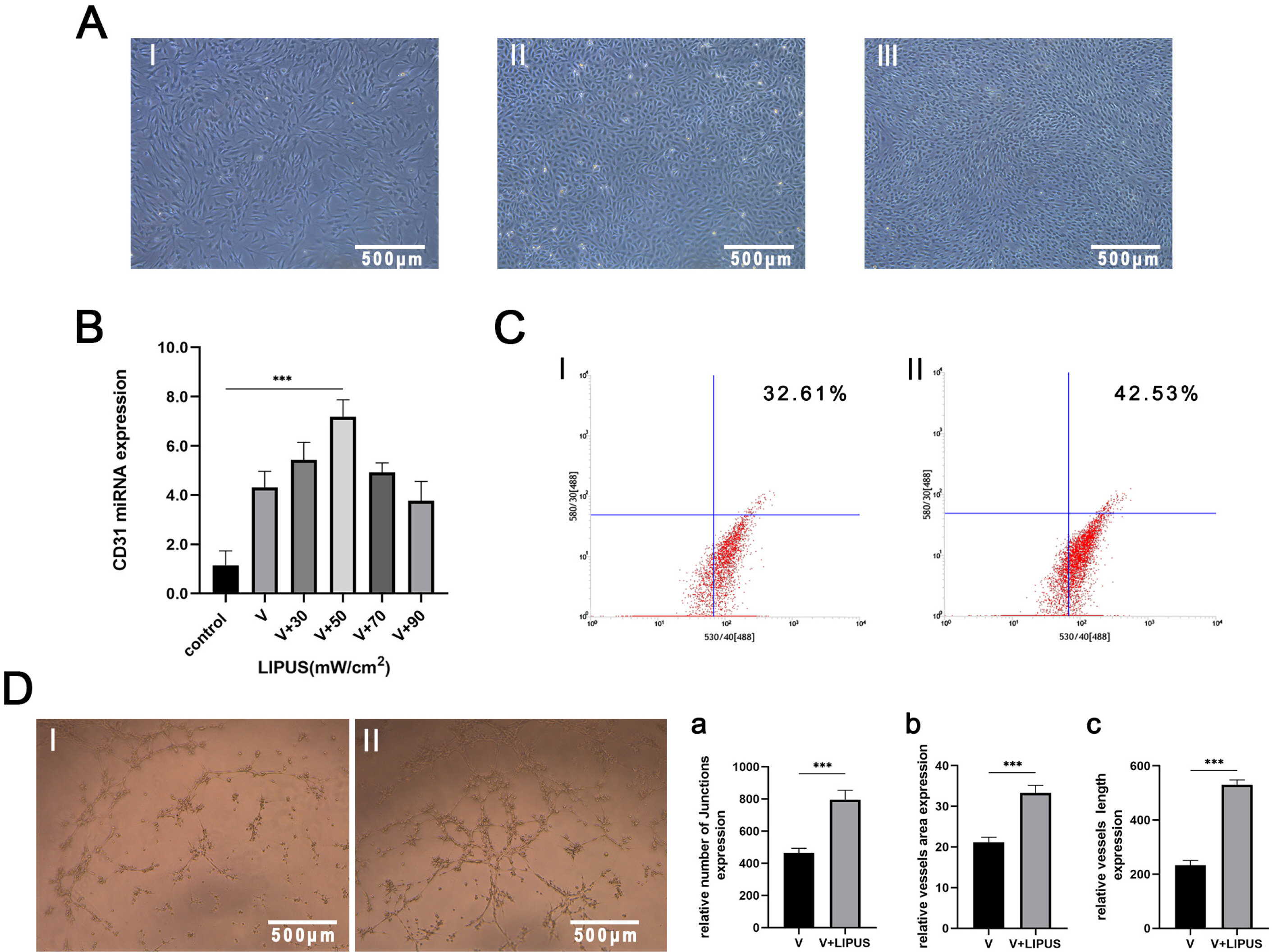
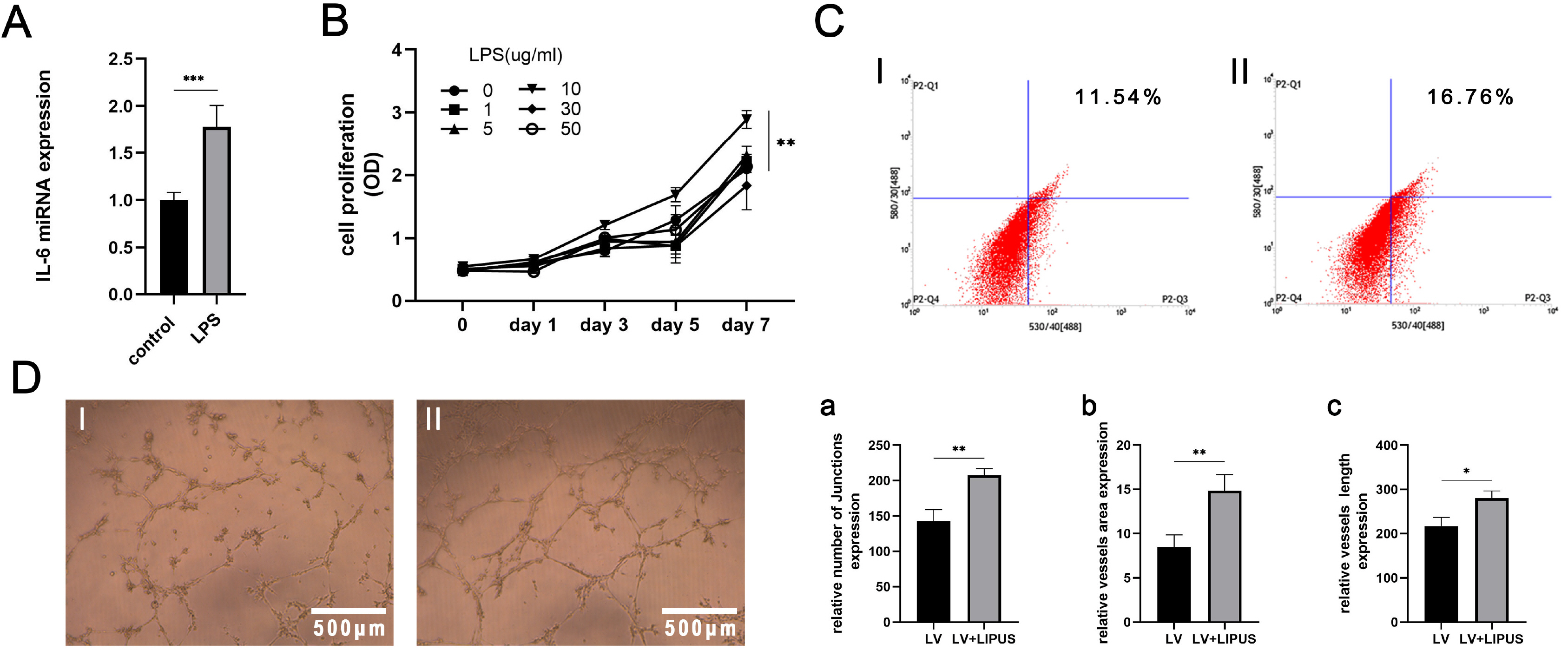
PDLSCs obtained from healthy people were used for endothelial induction, and 10 μg/ml lipopolysaccharide (LPS) was used to simulate the inflammatory state. The induced cells were treated with LIPUS (50 mW/cm2 , 1.5 MHz) to study its effect on the endothelial differentiation of PDLSCs and the tube formation of differentiated cells. PCR, flow cytometry, immunofluorescence, and Matrigel tube formation assays were used to detect the differentiation and tube formation of PDLSCs. GsMTx4 was used to inhibit the expression of Piezo1, and the role of the Piezo1 pathway in the endothelial differentiation and microvascular formation of PDLSCs after LIPUS treatment was studied. The data showed that LIPUS increased endothelial differentiation and angiogenesis in PDLSCs under inflammatory or noninflammatory conditions. The use of an inhibitor weakened the effect of LIPUS.
Conclusions
This study demonstrated that LIPUS can activate the expression of Piezo1 and promote the endothelial differentiation and microvascular formation of PDLSCs.
Keyword
Figure
Reference
-
References
1. Iwasaki K, Komaki M, Yokoyama N, Tanaka Y, Taki A, Honda I, Kimura Y, Takeda M, Akazawa K, Oda S, Izumi Y, Morita I. 2014; Periodontal regeneration using periodontal ligament stem cell-transferred amnion. Tissue Eng Part A. 20:693–704. DOI: 10.1089/ten.tea.2013.0017. PMID: 24032400. PMCID: PMC3926144. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84894141569&origin=inward.
Article2. Suphasiriroj W, Mikami M, Sato S. 2013; Comparative studies on microvascular endothelial cells isolated from periodontal tissue. J Periodontol. 84:1002–1009. DOI: 10.1902/jop.2012.120453. PMID: 23003919. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84880071289&origin=inward.
Article3. Komaki M. 2019; Pericytes in the periodontal ligament. Adv Exp Med Biol. 1122:169–186. DOI: 10.1007/978-3-030-11093-2_10. PMID: 30937869. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064213365&origin=inward.
Article4. Amorim BR, Silvério KG, Casati MZ, Sallum EA, Kantovitz KR, Nociti FH Jr. 2016; Neuropilin controls endothelial differentiation by mesenchymal stem cells from the periodontal ligament. J Periodontol. 87:e138–e147. DOI: 10.1902/jop.2016.150603. PMID: 26962679. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84977098349&origin=inward.
Article5. Wang L, Zhou F, Zhang P, Wang H, Qu Z, Jia P, Yao Z, Shen G, Li G, Zhao G, Li J, Mao Y, Xie Z, Xu W, Xu Y, Xu Y. 2017; Human type H vessels are a sensitive biomarker of bone mass. Cell Death Dis. 8:e2760. DOI: 10.1038/cddis.2017.36. PMID: 28471445. PMCID: PMC5520742. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85030319237&origin=inward.
Article6. Zhao Y, Xie L. 2020; Unique bone marrow blood vessels couple angiogenesis and osteogenesis in bone homeostasis and diseases. Ann N Y Acad Sci. 1474:5–14. DOI: 10.1111/nyas.14348. PMID: 32242943. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85094904629&origin=inward.
Article7. Xu R, Yallowitz A, Qin A, Wu Z, Shin DY, Kim JM, Debnath S, Ji G, Bostrom MP, Yang X, Zhang C, Dong H, Kermani P, Lalani S, Li N, Liu Y, Poulos MG, Wach A, Zhang Y, Inoue K, Di Lorenzo A, Zhao B, Butler JM, Shim JH, Glimcher LH, Greenblatt MB. 2018; Targeting skeletal endothelium to ameliorate bone loss. Nat Med. 24:823–833. DOI: 10.1038/s41591-018-0020-z. PMID: 29785024. PMCID: PMC5992080. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85047216276&origin=inward.
Article8. Gao Q, Walmsley AD, Cooper PR, Scheven BA. 2016; Ultrasound stimulation of different dental stem cell populations: role of mitogen-activated protein kinase signaling. J Endod. 42:425–431. DOI: 10.1016/j.joen.2015.12.019. PMID: 26830427. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84958967316&origin=inward.
Article9. Kusuyama J, Bandow K, Shamoto M, Kakimoto K, Ohnishi T, Matsuguchi T. 2014; Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J Biol Chem. 289:10330–10344. DOI: 10.1074/jbc.M113.546382. PMID: 24550383. PMCID: PMC4036157. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84898645115&origin=inward.
Article10. Wang Y, Jiang L, Xu T, Su Z, Guo X, Tu J, Zhang D, Sun W, Kong X. 2019; p38 MAPK signaling is a key mediator for low-intensity pulsed ultrasound (LIPUS) in cultured human omental adipose-derived mesenchymal stem cells. Am J Transl Res. 11:418–429. PMID: 30787998. PMCID: PMC6357340.11. Kang PL, Huang HH, Chen T, Ju KC, Kuo SM. 2019; Angiogenesis-promoting effect of LIPUS on hADSCs and HUVECs cultured on collagen/hyaluronan scaffolds. Mater Sci Eng C Mater Biol Appl. 102:22–33. DOI: 10.1016/j.msec.2019.04.045. PMID: 31146993. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85064273181&origin=inward.
Article12. Gorick CM, Chappell JC, Price RJ. 2019; Applications of ultrasound to stimulate therapeutic revascularization. Int J Mol Sci. 20:3081. DOI: 10.3390/ijms20123081. PMID: 31238531. PMCID: PMC6627741. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85068835794&origin=inward.
Article13. Su Z, Xu T, Wang Y, Guo X, Tu J, Zhang D, Kong X, Sheng Y, Sun W. 2019; Low-intensity pulsed ultrasound promotes apoptosis and inhibits angiogenesis via p38 signaling‑mediated endoplasmic reticulum stress in human endothelial cells. Mol Med Rep. 19:4645–4654. DOI: 10.3892/mmr.2019.10136. PMID: 30957188. PMCID: PMC6522835. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85066149040&origin=inward.
Article14. Ichijo S, Shindo T, Eguchi K, Monma Y, Nakata T, Morisue Y, Kanai H, Osumi N, Yasuda S, Shimokawa H. 2021; Low-intensity pulsed ultrasound therapy promotes recovery from stroke by enhancing angio-neurogenesis in mice in vivo. Sci Rep. 11:4958. DOI: 10.1038/s41598-021-84473-6. PMID: 33654156. PMCID: PMC7925563. PMID: ffd56aa231b04d448249e6d451f7840b. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85101938794&origin=inward.
Article15. Pei F, Liu J, Zhang L, Pan X, Huang W, Cen X, Huang S, Jin Y, Zhao Z. 2021; The functions of mechanosensitive ion channels in tooth and bone tissues. Cell Signal. 78:109877. Erratum in: Cell Signal 2022;92:110276. DOI: 10.1016/j.cellsig.2022.110276. PMID: 35124167. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85124601003&origin=inward.
Article16. Shen Y, Pan Y, Guo S, Sun L, Zhang C, Wang L. 2020; The roles of mechanosensitive ion channels and associated downstream MAPK signaling pathways in PDLC mechanotrans-duction. Mol Med Rep. 21:2113–2122. DOI: 10.3892/mmr.2020.11006. PMID: 32323761. PMCID: PMC7115221. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85082476072&origin=inward.
Article17. Li H, Deng Y, Tan M, Feng G, Kuang Y, Li J, Song J. 2020; Low-intensity pulsed ultrasound upregulates osteogenesis under inflammatory conditions in periodontal ligament stem cells through unfolded protein response. Stem Cell Res Ther. 11:215. DOI: 10.1186/s13287-020-01732-5. PMID: 32493507. PMCID: PMC7268771. PMID: 56de37e059cc4cb0a197371def1eab86. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85085973795&origin=inward.
Article18. Watson EC, Adams RH. 2018; Biology of bone: the vasculature of the skeletal system. Cold Spring Harb Perspect Med. 8:a031559. DOI: 10.1101/cshperspect.a031559. PMID: 28893838. PMCID: PMC6027931. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85049520284&origin=inward.
Article19. Bae YK, Kim GH, Lee JC, Seo BM, Joo KM, Lee G, Nam H. 2017; The significance of SDF-1α-CXCR4 axis in in vivo angiogenic ability of human periodontal ligament stem cells. Mol Cells. 40:386–392. DOI: 10.14348/molcells.2017.0004. PMID: 28614918. PMCID: PMC5523014. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85029515120&origin=inward.
Article20. Ye J, Zhang R, Chu Y, Li J, Wang M, Chen F, Wang X, Liu L, Wang Q. 2014; A comparative study on the endothelial cell differentiation of human healthy and inflammatory periodontal ligament stem cells. Chin J Conserv Dent. 24:7–12. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85082476072&origin=inward.21. Wei W, An Y, An Y, Fei D, Wang Q. 2018; Activation of autophagy in periodontal ligament mesenchymal stem cells promotes angiogenesis in periodontitis. J Periodontol. 89:718–727. DOI: 10.1002/JPER.17-0341. PMID: 29607508.
Article22. Kukolj T, Trivanović D, Djordjević IO, Mojsilović S, Krstić J, Obradović H, Janković S, Santibanez JF, Jauković A, Bugarski D. 2018; Lipopolysaccharide can modify differentiation and immunomodulatory potential of periodontal ligament stem cells via ERK1,2 signaling. J Cell Physiol. 233:447–462. DOI: 10.1002/jcp.25904. PMID: 28295277. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85019392694&origin=inward.
Article23. Kusuyama J, Nakamura T, Ohnishi T, Eiraku N, Noguchi K, Matsuguchi T. 2017; Low-intensity pulsed ultrasound (LIPUS) promotes BMP9-induced osteogenesis and suppresses inflammatory responses in human periodontal ligament-de-rived stem cells. J Orthop Trauma. 31:S4. DOI: 10.1097/01.bot.0000520897.92470.70. PMID: 28632668. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85046261594&origin=inward.
Article24. Ranade SS, Syeda R, Patapoutian A. 2015; Mechanically activated ion channels. Neuron. 87:1162–1179. Erratum in: Neuron 2015;88:433. DOI: 10.1016/j.neuron.2015.08.032. PMID: 26402601. PMCID: PMC4582600. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84942134031&origin=inward.
Article25. Kang H, Hong Z, Zhong M, Klomp J, Bayless KJ, Mehta D, Karginov AV, Hu G, Malik AB. 2019; Piezo1 mediates angiogenesis through activation of MT1-MMP signaling. Am J Physiol Cell Physiol. 316:C92–C103. DOI: 10.1152/ajpcell.00346.2018. PMID: 30427721. PMCID: PMC6383143. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85059798723&origin=inward.
Article26. Zhang L, Liu X, Gao L, Ji Y, Wang L, Zhang C, Dai L, Liu J, Ji Z. 2020; Activation of Piezo1 by ultrasonic stimulation and its effect on the permeability of human umbilical vein endothelial cells. Biomed Pharmacother. 131:110796. DOI: 10.1016/j.biopha.2020.110796. PMID: 33152952. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85091584742&origin=inward.
Article27. Kang T, Huang S, Li P, Shi T, Yu Q, Duan Y. 2014; Piezos' expression in periodontal tissues of rats. Chin J Conserv Dent. 24:269–273. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85082476072&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Low-Intensity Pulsed Ultrasound Promotes BMP9 Induced Osteoblastic Differentiation in Rat Dedifferentiated Fat Cells
- Comparison of Gene Expression from Supernumerary Dental Pulp and Periodontal Ligament Stem Cells
- Stem cell properties of cells derived from canine periodontal ligament
- Autologous Stem Cell Application in Periodontal Regeneration Technique (SAI-PRT) Using PDLSCs Directly From an Extracted Tooth...An Insight
- Skeletal myogenic differentiation of human periodontal ligament stromal cells isolated from orthodontically extracted premolars