J Korean Neurosurg Soc.
2022 Nov;65(6):861-867. 10.3340/jkns.2020.0339.
Preliminary Study on Natural Killer Cell Activity for Interferon-Gamma Production after Gamma Knife Radiosurgery for Brain Tumors
- Affiliations
-
- 1Department of Neurosurgery, Gachon University Gil Medical Center, Incheon, Korea
- 2Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea
- 3Cancer Research Institute, Seoul National University, Seoul, Korea
- 4Ischemia/Hypoxia Disease Institute, Seoul National University, Seoul, Korea
- 5Department of Neurosurgery, Soonchunhyang University Hospital, Seoul, Korea
- 6Department of Neurosurgery, Pusan National University Hospital, Busan, Korea
- 7Department of R & D, NK MAX Company, Sungnam, Korea
- KMID: 2535847
- DOI: http://doi.org/10.3340/jkns.2020.0339
Abstract
Objective
: High-dose radiation is well known to induce and modulate the immune system. This study was performed to evaluate the correlation between clinical outcomes and changes in natural killer cell activity (NKA) after Gamma Knife Radiosurgery (GKS) in patients with brain cancer.
Methods
: We performed an open-label, prospective, cross-sectional study of 38 patients who were treated with GKS for brain tumors, including metastatic and benign brain tumors. All of the patients underwent GKS, and blood samples were collected before and after GKS. NKA was measured using an enzyme-linked immunosorbent assay kit, to measure interferon-gamma (IFNγ) secreted by ex vivo-stimulated NK cells from whole blood. We explored the correlations between NK cell-produced IFNγ (NKA-IFNγ) levels and clinical parameters of patients who were treated with GKS for brain tumors.
Results
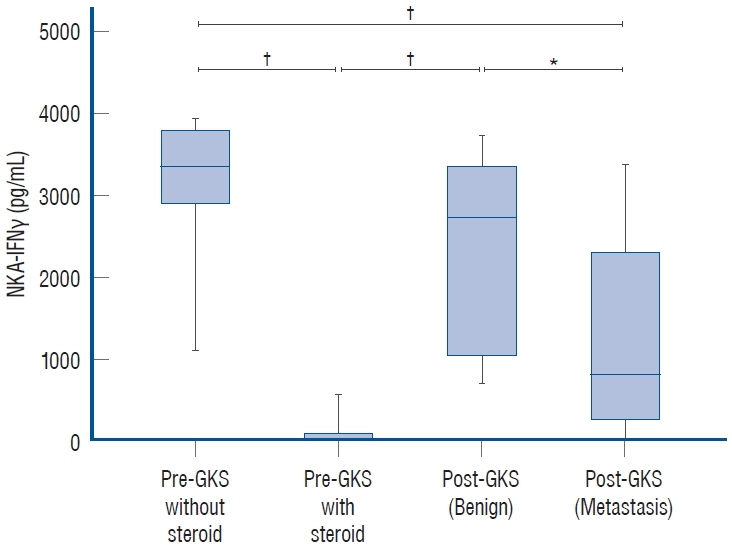
: NKA-IFNγ levels were decreased in metastatic brain tumor patients compared to those with benign brain tumors (p<0.0001). All the patients who used steroid treatment to reduce brain swelling after GKS had an NKA-IFNγ level of zero except one patient. High NKA-IFNγ levels were not associated with a rapid decrease in brain metastasis and did not increase after GKS.
Conclusion
: The activity of NK cells in metastatic brain tumors decreased more than that in benign brain tumors after GKS.
Figure
Reference
-
References
1. Ahn MJ, Lee K, Lee KH, Kim JW, Kim IY, Bae WK. Combination of antiPD-1 therapy and stereotactic radiosurgery for a gastric cancer patient with brain metastasis: a case report. BMC Cancer. 18:173. 2018.2. Barkin J, Rodriguez-Suarez R, Betito K. Association between natural killer cell activity and prostate cancer: a pilot study. Can J Uro. 24:8708–8713. 2017.3. Berghoff AS, Preusser M. The inflammatory microenvironment in brain metastases: potential treatment target? Chin Clin Oncol. 4:21. 2015.4. Caligiuri MA. Human natural killer cells. Blood. 112:461–469. 2008.5. Carvalho HA, Villar RC. Radiotherapy and immune response: the systemic effects of a local treatment. Clinics (Sao Paulo). 73(suppl 1):e557s. 2018.6. Chen J, Liu X, Zeng Z, Li J, Luo Y, Sun W, et al. Immunomodulation of NK cells by ionizing radiation. Front Oncol. 10:874. 2020.7. Cho D, Campana D. Expansion and activation of natural killer cells for cancer immunotherapy. Korean J Lab Med. 29:89–96. 2009.8. Dahlberg CI, Sarhan D, Chrobok M, Duru AD, Alici E. Natural killer cellbased therapies targeting cancer: possible strategies to gain and sustain anti-tumor activity. Front Immunol. 6:605. 2015.9. Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res. 15:5379–5388. 2009.10. Dix AR, Brooks WH, Roszman TL, Morford LA. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 100:216–232. 1999.11. Frey B, Rubner Y, Kulzer L, Werthmöller N, Weiss EM, Fietkau R, et al. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol Immunother. 63:29–36. 2014.12. Hatiboglu MA, Kocyigit A, Guler EM, Nalli A, Akdur K, Sakarcan A, et al. Gamma knife radiosurgery compared to whole brain radiation therapy enhances immunity via immunoregulatory molecules in patients with metastatic brain tumours. Br J Neurosurg. 34:604–610. 2020.13. Jeong H, Bok S, Hong BJ, Choi HS, Ahn GO. Radiation-induced immune responses: mechanisms and therapeutic perspectives. Blood Res. 51:157–163. 2016.14. Jobin G, Rodriguez-Suarez R, Betito K. Association between natural killer cell activity and colorectal cancer in high-risk subjects undergoing colonoscopy. Gastroenterology. 153:980–987. 2017.15. Jung YS, Kwon MJ, Park DI, Sohn CI, Park JH. Association between natural killer cell activity and the risk of colorectal neoplasia. J Gastroenterol Hepatol. 33:831–836. 2018.16. Kaur P, Asea A. Radiation-induced effects and the immune system in cancer. Front Oncol. 2:191. 2012.17. Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, DvirSzternfeld R, Ulland TK, et al. A unique Microglia type associated with restricting development of alzheimer’s disease. Cell. 169:1276–1290.e17. 2017.18. Kipnis J, Filiano AJ. Neuroimmunology in 2017: the central nervous system: privileged by immune connections. Nat Rev Immunol. 18:83–84. 2018.19. Koo KC, Shim DH, Yang CM, Lee SB, Kim SM, Shin TY, et al. Reduction of the CD16(-)CD56bright NK cell subset precedes NK cell dysfunction in prostate cancer. PLoS One. 8:e78049. 2013.20. Lee J, Park KH, Ryu JH, Bae HJ, Choi A, Lee H, et al. Natural killer cell activity for IFN-gamma production as a supportive diagnostic marker for gastric cancer. Oncotarget. 8:70431–70440. 2017.21. Lee SB, Cha J, Kim IK, Yoon JC, Lee HJ, Park SW, et al. A high-throughput assay of NK cell activity in whole blood and its clinical application. Biochem Biophys Res Commun. 445:584–590. 2014.22. Lee SJ, Kang WY, Yoon Y, Jin JY, Song HJ, Her JH, et al. Natural killer (NK) cells inhibit systemic metastasis of glioblastoma cells and have therapeutic effects against glioblastomas in the brain. BMC Cancer. 15:1011. 2015.23. Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 523:337–341. 2015.24. Luetke-Eversloh M, Killig M, Romagnani C. Signatures of human NK cell development and terminal differentiation. Front Immunol. 4:499. 2013.25. McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, et al. A sense of danger from radiation. Radiat Res. 162:1–19. 2004.26. McKelvey KJ, Hudson AL, Back M, Eade T, Diakos CI. Radiation, inflammation and the immune response in cancer. Mamm Genome. 29:843–865. 2018.27. Nederby L, Hansen T, Raunkilde L, Jensen LH, Jakobsen AKM. Natural killer cell activity: a test for immune reactivity with clinical perspectives. JCO. 36 5_suppl:87–2018.28. Nederby L, Jakobsen A, Hokland M, Hansen TF. Quantification of NK cell activity using whole blood: methodological aspects of a new test. J Immunol Methods. 458:21–25. 2018.29. Nordmann N, Hubbard M, Nordmann T, Sperduto PW, Clark HB, Hunt MA. Effect of gamma knife radiosurgery and programmed cell death 1 receptor antagonists on metastatic melanoma. Cureus. 9:e1943. 2017.30. Parney IF. Basic concepts in glioma immunology. Adv Exp Med Biol. 746:42–52. 2012.31. Poli A, Wang J, Domingues O, Planagumà J, Yan T, Rygh CB, et al. Targeting glioblastoma with NK cells and mAb against NG2/CSPG4 prolongs animal survival. Oncotarget. 4:1527–1546. 2013.32. Schoenhals JE, Seyedin SN, Anderson C, Brooks ED, Li YR, Younes AI, et al. Uncovering the immune tumor microenvironment in non-small cell lung cancer to understand response rates to checkpoint blockade and radiation. Transl Lung Cancer Res. 6:148–158. 2017.33. Servadei F, Parente R, Bucci M, Beltrandi E, Tognetti F, Gaist G. Particular features of cell-mediated immunity in patients with anaplastic gliomas. A comparison with kidney and bladder cancer patients. J Neurooncol. 1:327–332. 1983.34. Sologuren I, Rodríguez-Gallego C, Lara PC. Immune effects of high dose radiation treatment: implications of ionizing radiation on the development of bystander and abscopal effects. Transl Canc Res. 3:18–31. 2014.35. Stevens A, Klöter I, Roggendorf W. Inflammatory infiltrates and natural killer cell presence in human brain tumors. Cancer. 61:738–743. 1988.36. Szeifert GT, Salmon I, Rorive S, Massager N, Devriendt D, Simon S, et al. Does gamma knife surgery stimulate cellular immune response to metastatic brain tumors? A histopathological and immunohistochemical study. J Neurosurg. 102 Suppl:180–184. 2005.37. Vivier E, Ugolini S. Natural killer cells: from basic research to treatments. Front Immunol. 2:18. 2011.38. Wallin RP, Screpanti V, Michaëlsson J, Grandien A, Ljunggren HG. Regulation of perforin-independent NK cell-mediated cytotoxicity. Eur J Immunol. 33:2727–2735. 2003.39. Zhang T, Yu H, Ni C, Zhang T, Liu L, Lv Q, et al. Hypofractionated stereotactic radiation therapy activates the peripheral immune response in operable stage I non-small-cell lung cancer. Sci Rep. 7:4866. 2017.40. Zhi-Feng W, Le-Yuan Z, Xiao-Hui Z, Ya-Bo G, Jian-Ying Z, Yong H, et al. TLR4-dependent immune response promotes radiation-induced liver disease by changing the liver tissue interstitial microenvironment during liver cancer radiotherapy. Radiat Res. 182:674–682. 2014.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- How to use Leksell GammaPlan
- Stereotactic radiosurgery for brain metastases
- Normal pressure hydrocephalus after gamma knife radiosurgery in a patient with vestibular schwannoma
- Postoperative Residual Juvenile Nasopharyngeal Angiofibroma Treated with Gamma Knife Surgery
- Clinical Analysis of Gamma Knife Radiosurgery for Brain Metastases