Anesth Pain Med.
2022 Jul;17(3):312-319. 10.17085/apm.22136.
Improvement of compliance to the Portland intensive insulin therapy during liver transplantation after introducing an application software: a retrospective single center cohort study
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Korea Cancer Center Hospital, Seoul, Korea
- 2Department of Anesthesiology and Pain Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2535325
- DOI: http://doi.org/10.17085/apm.22136
Abstract
- Background
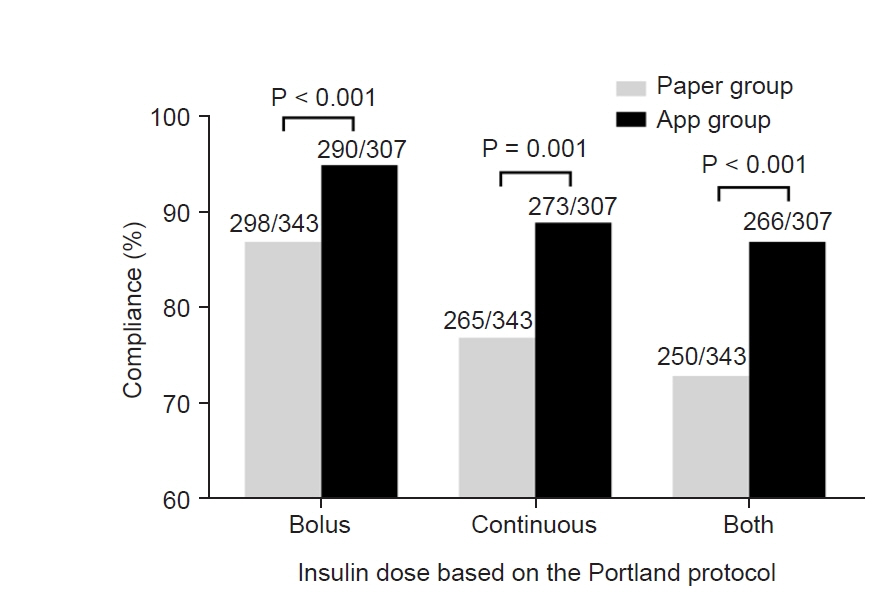
The Portland intensive insulin therapy effectively controls acute hyperglycemic change after graft reperfusion during liver transplantation. However, the time-consuming sophistication acts as a barrier leading to misinterpretation and decreasing compliance to the protocol; thus, we newly introduced an application software “Insulin protocol calculator” which automatically calculates therapeutic bolus/continuous insulin doses based on the Portland protocol. Methods: Of 144 patients who underwent liver transplantation, 74 patients were treated before the introduction of “Insulin protocol calculator” by using a paper manual, and 70 patients were treated by using the application. Compliance was defined as the proportion of patients treated with exact bolus/continuous insulin dose according to the Portland protocol. Results: Compliance was significantly greater in app group than in paper group regarding bolus dose (94.5% and 86.9%, P < 0.001), continuous dose (88.9% and 77.3%, P = 0.001), and both doses (86.6% and 73.8%, P < 0.001). Blood glucose concentration was significantly lower in app group at 3 h (125 ± 17 mg/dl vs. 136 ± 19 mg/dl, P = 0.014) and 4 h (135 ± 22 mg/dl vs. 115 ± 15 mg/dl, P = 0.029) after graft reperfusion. Acute hyperglycemic change during 30 min was more prominent in app group while hyperglycemia incidence was 71.4% vs. 54.1% (P = 0.031). However, hyperglycemia risk was comparable at 2 h (31.4% vs. 31.1%, P = 0.964), and even insignificantly lower in app group at 3 h (7.1% vs. 19.5%, P = 0.184). Conclusions: Compliance to the Portland protocol was significantly improved after introducing the application software; post-reperfusion hyperglycemia was better controlled. “Insulin protocol calculator” is cost-effective and time-saving with potential clinical benefits
Keyword
Figure
Reference
-
1. Han S, Sangwook Ko J, Jin SM, Man Kim J, Choi SJ, Joh JW, et al. Glycemic responses to intermittent hepatic inflow occlusion in living liver donors. Liver Transpl. 2015; 21:180–6.
Article2. Han S, Ko JS, Jin SM, Park HW, Kim JM, Joh JW, et al. Intraoperative hyperglycemia during liver resection: predictors and association with the extent of hepatocytes injury. PLoS One. 2014; 9:e109120.
Article3. Turina M, Fry DE, Polk HC Jr. Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005; 33:1624–33.
Article4. Jafar N, Edriss H, Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am J Med Sci. 2016; 351:201–11.
Article5. Behrends M, Martinez-Palli G, Niemann CU, Cohen S, Ramachandran R, Hirose R. Acute hyperglycemia worsens hepatic ischemia/reperfusion injury in rats. J Gastrointest Surg. 2010; 14:528–35.
Article6. Park C, Hsu C, Neelakanta G, Nourmand H, Braunfeld M, Wray C, et al. Severe intraoperative hyperglycemia is independently associated with surgical site infection after liver transplantation. Transplantation. 2009; 87:1031–6.
Article7. Ammori JB, Sigakis M, Englesbe MJ, O'Reilly M, Pelletier SJ. Effect of intraoperative hyperglycemia during liver transplantation. J Surg Res. 2007; 140:227–33.
Article8. Park CS. Predictive roles of intraoperative blood glucose for post-transplant outcomes in liver transplantation. World J Gastroenterol. 2015; 21:6835–41.
Article9. Kang R, Han S, Lee KW, Kim GS, Choi SJ, Ko JS, et al. Portland intensive insulin therapy during living donor liver transplantation: association with postreperfusion hyperglycemia and clinical outcomes. Sci Rep. 2018; 8:16306.
Article10. Kang R, Han S, Kim JM, Lee KW, Park HW, Ahn JH, et al. Postoperative hyperglycemia may negatively impact cytomegalovirus infection in seropositive liver transplant recipients: a retrospective cohort study. Transpl Int. 2020; 33:68–75.
Article11. Furnary AP, Gao G, Grunkemeier GL, Wu Y, Zerr KJ, Bookin SO, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003; 125:1007–21.
Article12. Furnary AP, Wu Y, Bookin SO. Effect of hyperglycemia and continuous intravenous insulin infusions on outcomes of cardiac surgical procedures: the Portland Diabetic Project. Endocr Pract. 2004; 10 Suppl 2:21–33.
Article13. Furnary AP, Cheek DB, Holmes SC, Howell WL, Kelly SP. Achieving tight glycemic control in the operating room: lessons learned from 12 years in the trenches of a paradigm shift in anesthetic care. Semin Thorac Cardiovasc Surg. 2006; 18:339–45.
Article14. Han S, Park HW, Song JH, Gwak MS, Lee WJ, Kim G, et al. Association between intraoperative platelet transfusion and early graft regeneration in living donor liver transplantation. Ann Surg. 2016; 264:1065–72.
Article15. Battezzati A, Caumo A, Martino F, Sereni LP, Coppa J, Romito R, et al. Nonhepatic glucose production in humans. Am J Physiol Endocrinol Metab. 2004; 286:E129–35.
Article16. De Wolf A, Frenette L, Kang Y, Tang C. Insulin decreases the serum potassium concentration during the anhepatic stage of liver transplantation. Anesthesiology. 1993; 78:677–82.
Article17. Steil GM, Deiss D, Shih J, Buckingham B, Weinzimer S, Agus MS. Intensive care unit insulin delivery algorithms: why so many? How to choose? J Diabetes Sci Technol. 2009; 3:125–40.
Article18. Han S, Lee JH, Kim G, Ko JS, Choi SJ, Kwon JH, et al. Bioreactance is not interchangeable with thermodilution for measuring cardiac output during adult liver transplantation. PLoS One. 2015; 10:e0127981.
Article19. Han S, Kwon JH, Jung SH, Seo JY, Jo YJ, Jang JS, et al. Perioperative fresh red blood cell transfusion may negatively affect recipient survival after liver transplantation. Ann Surg. 2018; 267:346–51.
Article20. Han S, Kim G, Ko JS, Sinn DH, Yang JD, Joh JW, et al. Safety of the use of blood salvage and autotransfusion during liver transplantation for hepatocellular carcinoma. Ann Surg. 2016; 264:339–43.
Article21. Kwon JH, Han S, Kim D, Kuk JH, Cho H, Kim S, et al. Blood salvage and autotransfusion does not increase the risk of tumor recurrence after liver transplantation for advanced hepatocellular carcinoma. Ann Surg;2021. doi: 10.1097/SLA.0000000000004866. [Epub ahead of print].22. Kwon JH, Han S, Jang JS, Lee KW, Ahn JH, Kim K, et al. Decrease in the risk of posttransplant hepatocellular carcinoma recurrence after the conversion to prestorage leukoreduction for transfused red blood cells. Transplantation. 2021; 105:577–85.
Article23. Park MH, Shim HS, Kim WH, Kim HJ, Kim DJ, Lee SH, et al. Clinical risk scoring models for prediction of acute kidney injury after living donor liver transplantation: a retrospective observational study. PLoS One. 2015; 10:e0136230.
Article24. Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006; 295:1681–7.
Article25. Krinsley JS. Glycemic variability and mortality in critically ill patients: the impact of diabetes. J Diabetes Sci Technol. 2009; 3:1292–301.
Article26. Duncan AE, Abd-Elsayed A, Maheshwari A, Xu M, Soltesz E, Koch CG. Role of intraoperative and postoperative blood glucose concentrations in predicting outcomes after cardiac surgery. Anesthesiology. 2010; 112:860–71.
Article27. Bates DW, Teich JM, Lee J, Seger D, Kuperman GJ, Ma'Luf N, et al. The impact of computerized physician order entry on medication error prevention. J Am Med Inform Assoc. 1999; 6:313–21.
Article28. Vanhorebeek I, De Vos R, Mesotten D, Wouters PJ, De Wolf-Peeters C, Van den Berghe G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet. 2005; 365:53–9.29. Han S, Kim G, Lee SK, Kwon CH, Gwak M, Lee S, et al. Comparison of the tolerance of hepatic ischemia/reperfusion injury in living donors: macrosteatosis versus microsteatosis. Liver Transpl. 2014; 20:775–83.
Article30. Noack K, Bronk SF, Kato A, Gores GJ. The greater vulnerability of bile duct cells to reoxygenation injury than to anoxia. Implications for the pathogenesis of biliary strictures after liver transplantation. Transplantation. 1993; 56:495–500.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intensive Insulin Therapy in Type 1 Diabetes
- Pancreas Transplantation
- The application of immunosuppressants education video and instant messaging software to improve compliance in transplant recipients
- Mesenchymal Stem Cell Therapy in Acute Liver Failure
- Current Status of Pediatric Liver Transplantation