J Yeungnam Med Sci.
2022 Oct;39(4):336-340. 10.12701/yujm.2021.01375.
Posterior reversible encephalopathy syndrome related to anemia correction in a patient with uterine myoma: a case report
- Affiliations
-
- 1Department of Obstetrics and Gynecology, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu, Korea
- KMID: 2534662
- DOI: http://doi.org/10.12701/yujm.2021.01375
Abstract
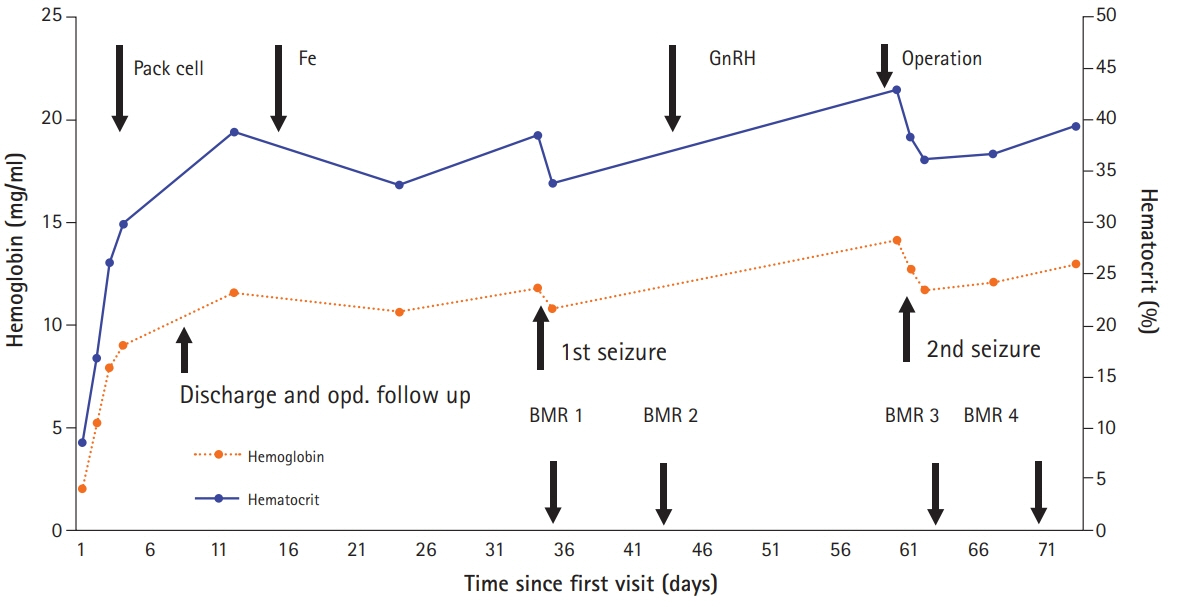
- Although posterior reversible encephalopathy syndrome (PRES) is induced by various causes, a few cases have occurred after severe anemia correction. In this case report, a 45-year-old female patient visited emergency department with a chief complaint of dizziness due to severe anemia related to hypermenorrhea caused by uterine myoma. Before her operation, she had an abrupt headache and seizure during anemia correction with transfusion and injection of gonadotropin-releasing hormone agonist. Immediately after the operation, she experienced visual disturbances, followed by limb weakness and tonic-clonic movements. Magnetic resonance imaging showed alterations in parietal and occipital lobes suggesting cerebrovascular edema with hypoperfusion. Here, we presented and discussed the clinical and radiologic features of PRES related to anemia correction.
Keyword
Figure
Reference
-
References
1. Zimmermann A, Bernuit D, Gerlinger C, Schaefers M, Geppert K. Prevalence, symptoms and management of uterine fibroids: an international internet-based survey of 21,746 women. BMC Womens Health. 2012; 12:6.
Article2. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996; 334:494–500.
Article3. Casey SO, Sampaio RC, Michel E, Truwit CL. Posterior reversible encephalopathy syndrome: utility of fluid-attenuated inversion recovery MR imaging in the detection of cortical and subcortical lesions. AJNR Am J Neuroradiol. 2000; 21:1199–206.4. McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, et al. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007; 189:904–12.
Article5. Magnano MD, Bush TM, Herrera I, Altman RD. Reversible posterior leukoencephalopathy in patients with systemic lupus erythematosus. Semin Arthritis Rheum. 2006; 35:396–402.
Article6. Dube M, Rathore R. Blood-transfusion-related posterior reversible encephalopathy syndrome - a description of a new case and review of the literature. Brain Circ. 2020; 6:269–73.
Article7. Nakamura Y, Sugino M, Tsukahara A, Nakazawa H, Yamamoto N, Arawaka S. Posterior reversible encephalopathy syndrome with extensive cytotoxic edema after blood transfusion: a case report and literature review. BMC Neurol. 2018; 18:190.
Article8. Singh K, Gupta R, Kamal H, Silvestri NJ, Wolfe GI. Posterior reversible encephalopathy syndrome secondary to blood transfusion. J Clin Neurosci. 2015; 22:592–4.
Article9. Lee M, Kim TH, Kim SJ, Jee BC. Posterior reversible encephalopathy syndrome in a woman who used gonadotropin-releasing hormone agonists: a case report. Obstet Gynecol Sci. 2019; 62:69–72.
Article10. Chakrabarti S, Morton JS, Davidge ST. Mechanisms of estrogen effects on the endothelium: an overview. Can J Cardiol. 2014; 30:705–12.
Article11. Provenzale JM, Petrella JR, Cruz LC Jr, Wong JC, Engelter S, Barboriak DP. Quantitative assessment of diffusion abnormalities in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2001; 22:1455–61.12. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008; 29:1043–9.
Article13. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008; 29:447–55.
Article14. Köhrmann M, Struffert T, Frenzel T, Schwab S, Doerfler A. The hyperintense acute reperfusion marker on fluid-attenuated inversion recovery magnetic resonance imaging is caused by gadolinium in the cerebrospinal fluid. Stroke. 2012; 43:259–61.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Posterior Reversible Encephalopathy Syndrome after Massive Blood Transfusion in a Normotensive Patient
- Posterior reversible encephalopathy syndrome in a woman who used gonadotropin-releasing hormone agonists: a case report
- A Case of Posterior Reversible Encephalopathy Syndrome with Bilateral Visual Impairment
- Posterior Reversible Encephalopathy Syndrome in a Patient with Intoxication of Arisaema amurense
- Posterior reversible encephalopathy syndrome and reversible cerebral vasoconstriction syndrome associated with acute exacerbation of chronic obstructive pulmonary disease