Cancer Res Treat.
2022 Oct;54(4):1191-1199. 10.4143/crt.2021.985.
Optimal Definition of Biochemical Recurrence in Patients Who Receive Salvage Radiotherapy Following Radical Prostatectomy for Prostate Cancer
- Affiliations
-
- 1The Proton Therapy Center, Research Institute and Hospital, National Cancer Center, Goyang, Korea
- 2Department of Radiation Oncology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 3Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 4Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 5Department of Radiation Oncology, Yongin Severance Hospital, Yonsei University College of Medicine, Yongin, Korea
- 6Department of Radiation Oncology, Chonnam National University Hwasun Hospital, Chonnam National University College of Medicine, Hwasun, Korea
- 7Department of Radiation Oncology, Ewha Womans University Medical Center, Ewha Womans University College of Medicine, Seoul, Korea
- 8Department of Radiation Oncology, Kyung Hee University Hospital, Kyung Hee University College of Medicine, Seoul, Korea
- 9Department of Radiation Oncology, Dong-A University Hospital, Dong-A University School of Medicine, Busan, Korea
- 10Biostatistics Collaboration Team, Research Institute and Hospital, National Cancer Center, Goyang, Korea
- 11Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2534198
- DOI: http://doi.org/10.4143/crt.2021.985
Abstract
- Purpose
This study proposed the optimal definition of biochemical recurrence (BCR) after salvage radiotherapy (SRT) following radical prostatectomy for prostate cancer.
Materials and Methods
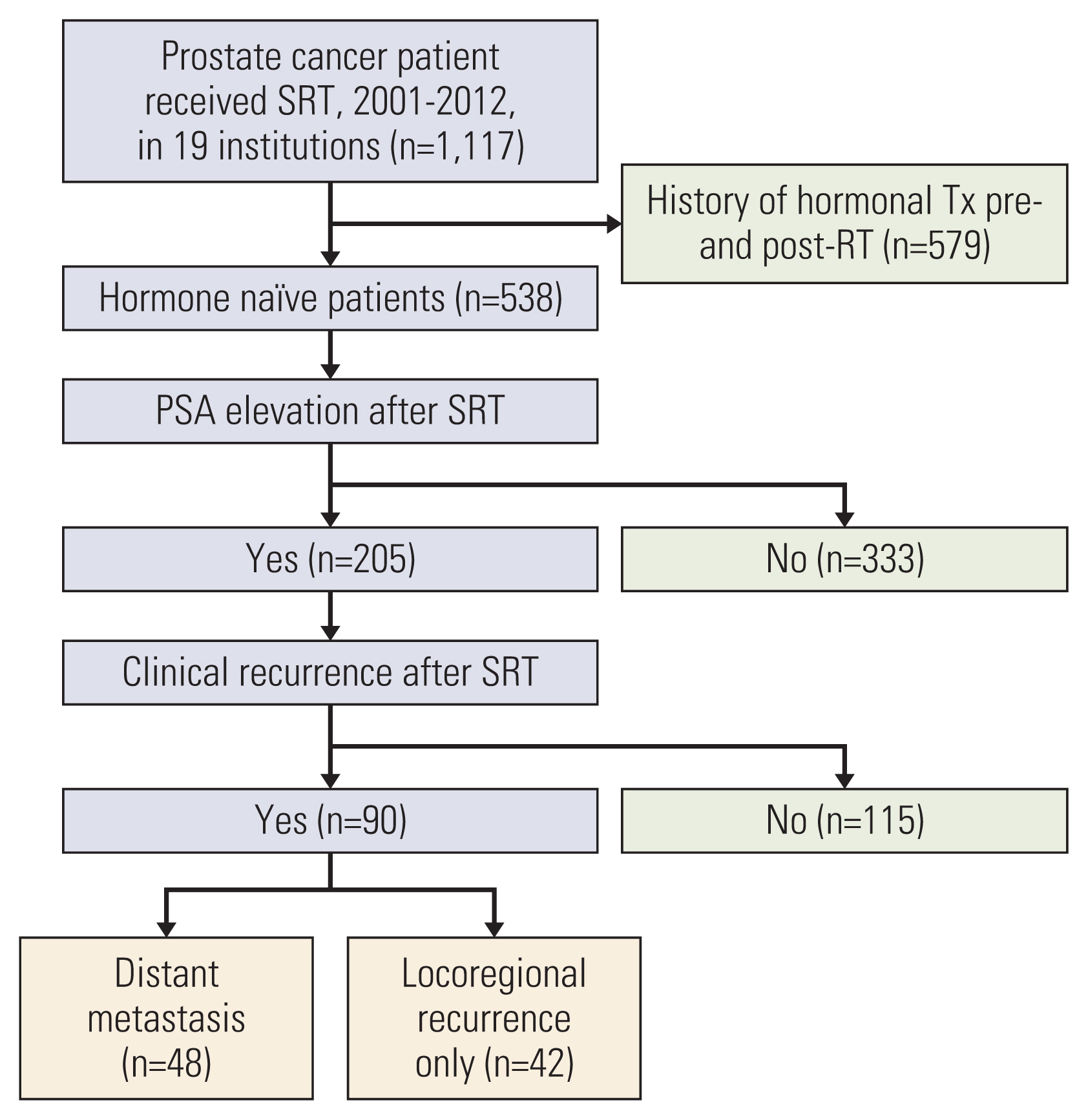
Among 1,117 patients who had received SRT, data from 205 hormone-naïve patients who experienced post-SRT prostate-specific antigen (PSA) elevation were included in a multi-institutional database. The primary endpoint was to determine the PSA parameters predictive of distant metastasis (DM). Absolute serum PSA levels and the prostate-specific antigen doubling time (PSA-DT) were adopted as PSA parameters.
Results
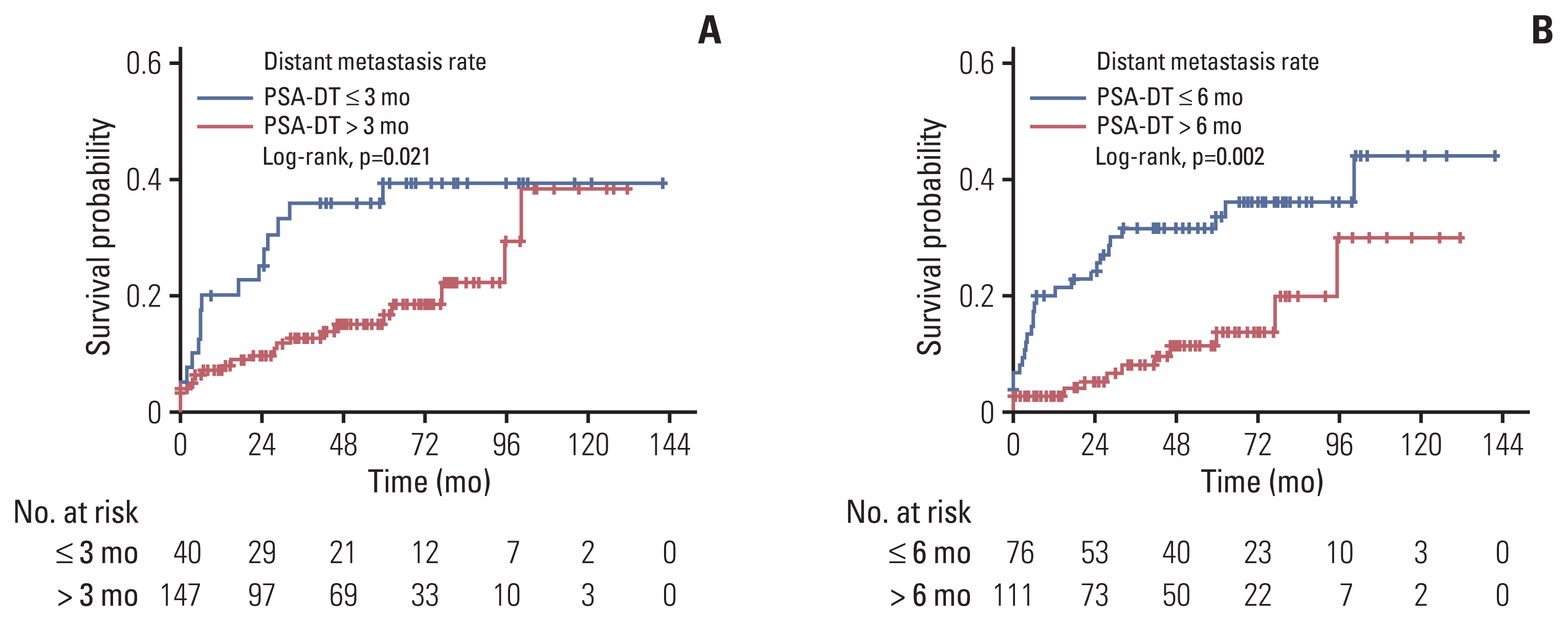
When BCR was defined based on serum PSA levels ranging from 0.4 ng/mL to nadir+2.0 ng/mL, the 5-year probability of DM was 27.6%-33.7%. The difference in the 5-year probability of DM became significant when BCR was defined as a serum PSA level of 0.8 ng/ml or higher (1.0-2.0 ng/mL). Application of a serum PSA level of ≥ 0.8 ng/mL yielded a c-index value of 0.589. When BCR was defined based on the PSA-DT, the 5-year probability was 22.7%-39.4%. The difference was significant when BCR was defined as a PSA-DT ≤ 3 months and ≤ 6 months. Application of a PSA-DT ≤ 6 months yielded the highest c-index (0.660). These two parameters complemented each other; for patients meeting both PSA parameters, the probability of DM was 39.5%-44.5%; for those not meeting either parameter, the probability was 0.0%-3.1%.
Conclusion
A serum PSA level > 0.8 ng/mL was a reasonable threshold for the definition of BCR after SRT. In addition, a PSA-DT ≤ 6 months was significantly predictive of subsequent DM, and combined application of both parameters enhanced predictability.
Figure
Reference
-
References
1. Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999; 281:1591–7.2. Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004; 172:910–4.3. Neuhof D, Hentschel T, Bischof M, Sroka-Perez G, Hohenfellner M, Debus J. Long-term results and predictive factors of three-dimensional conformal salvage radiotherapy for biochemical relapse after prostatectomy. Int J Radiat Oncol Biol Phys. 2007; 67:1411–7.4. Forman JD, Meetze K, Pontes E, Wood DP Jr, Shamsa F, Rana T, et al. Therapeutic irradiation for patients with an elevated post-prostatectomy prostate specific antigen level. J Urol. 1997; 158:1436–9.5. Lee SU, Cho KH, Park W, Cho WK, Kim JS, Wee CW, et al. Clinical outcomes of postoperative radiotherapy following radical prostatectomy in patients with localized prostate cancer: a multicenter retrospective sudy (KROG 18-01) of a Korean population. Cancer Res Treat. 2020; 52:167–80.6. D’Amico AV, Hanks GE. Linear regressive analysis using prostate-specific antigen doubling time for predicting tumor biology and clinical outcome in prostate cancer. Cancer. 1993; 72:2638–43.7. Stish BJ, Pisansky TM, Harmsen WS, Davis BJ, Tzou KS, Choo R, et al. Improved metastasis-free and survival outcomes with early salvage radiotherapy in men with detectable prostate-specific antigen after prostatectomy for prostate cancer. J Clin Oncol. 2016; 34:3864–71.8. Bernard JR Jr, Buskirk SJ, Heckman MG, Diehl NN, Ko SJ, Macdonald OK, et al. Salvage radiotherapy for rising prostate-specific antigen levels after radical prostatectomy for prostate cancer: dose-response analysis. Int J Radiat Oncol Biol Phys. 2010; 76:735–40.9. Shipley WU, Seiferheld W, Lukka HR, Major PP, Heney NM, Grignon DJ, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017; 376:417–28.10. Xie W, Regan MM, Buyse M, Halabi S, Kantoff PW, Sartor O, et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol. 2017; 35:3097–104.11. Gharzai LA, Jiang R, Wallington D, Jones G, Birer S, Jairath N, et al. Intermediate clinical endpoints for surrogacy in localised prostate cancer: an aggregate meta-analysis. Lancet Oncol. 2021; 22:402–10.12. Stephenson AJ, Kattan MW, Eastham JA, Dotan ZA, Bianco FJ Jr, Lilja H, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006; 24:3973–8.13. Patel A, Dorey F, Franklin J, deKernion JB. Recurrence patterns after radical retropubic prostatectomy: clinical usefulness of prostate specific antigen doubling times and log slope prostate specific antigen. J Urol. 1997; 158:1441–5.14. Hanks GE, D’Amico A, Epstein BE, Schultheiss TE. Prostatic-specific antigen doubling times in patients with prostate cancer: a potentially useful reflection of tumor doubling time. Int J Radiat Oncol Biol Phys. 1993; 27:125–7.15. Jackson WC, Johnson SB, Li D, Foster C, Foster B, Song Y, et al. A prostate-specific antigen doubling time of < 6 months is prognostic for metastasis and prostate cancer-specific death for patients receiving salvage radiation therapy post radical prostatectomy. Radiat Oncol. 2013; 8:170.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Salvage Radiotherapy for Patients with PSA Relapse Following Radical Prostatectomy: Issues and Challenges
- How can we best manage biochemical failure after radical prostatectomy?
- Hospital readmissions for patients with prostate cancer are higher after radiotherapy than after prostatectomy
- Outcome and Prognostic Factors of Salvage Radiotherapy for Biochemical Failure after Radical Prostatectomy: A Single Institute Experience
- Radical Prostatectomy