Ann Surg Treat Res.
2022 Oct;103(4):195-204. 10.4174/astr.2022.103.4.195.
Clinical use of perioperative magnetic resonance imaging-based breast volumetric analysis in final implant volume prediction for two-stage breast reconstruction
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, Ajou University School of Medicine, Suwon, Korea
- KMID: 2534119
- DOI: http://doi.org/10.4174/astr.2022.103.4.195
Abstract
- Purpose
Breast volume is an important factor in breast reconstruction; however, the surgeon is expected to deliver the volume expectation with his aesthetic inspiration. Therefore, objective volumetry must be developed. This study aimed to conduct an MRI-based breast volumetric analysis. With periodic analysis of 2-stage breast reconstruction, we suggest the possibility of clinical use of breast volumetry in implant volume prediction.
Methods
This retrospective study included 140 patients who underwent unilateral 2-stage breast reconstruction (tissue expander followed by implant insertion) between January 1, 2017 and December 31, 2019. The MRI image was converted into a 3-dimensional image with a reconstruction program (A-VIEW, Coreline Soft). MRI image was obtained before the surgery and then at 1, 3, 6, 12, and 24 months postoperatively. The volume was automatically calculated.
Results
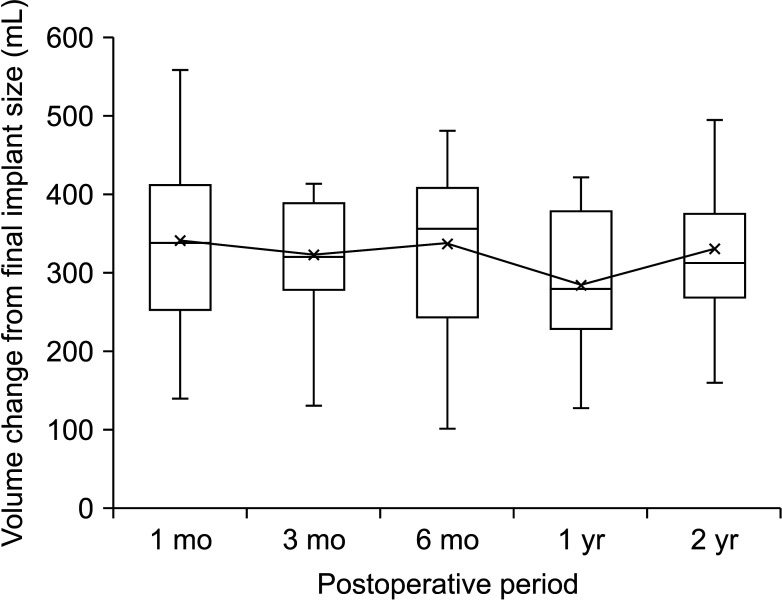
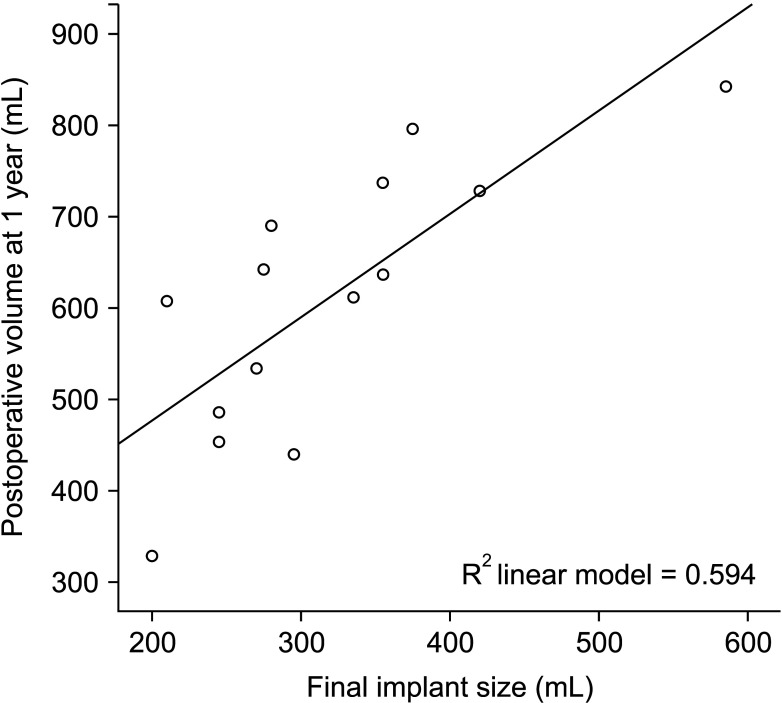
Compared with the preoperative volume, maximized volume and differences were noted at 1 month and minimized at 1 year. The correlation between MRI-based preoperative breast volumetry and the mastectomy specimen volume was 0.611. Volume difference between the MRI-based preoperative state and the implant volume showed a minimal difference at 1 year. The final implant size prediction formula was calculated using the 1-year postoperative volume (P < 0.001, R 2= 0.594).
Conclusion
To avoid breast reconstruction based solely on the surgeon’s subjective assessment, MRI-based breast volumetry could be a useful method to develop more scientific and objective breast reconstruction planning. We suggest a volume prediction formula that describes the relationship between the postoperative breast volume and the final breast implant size.
Figure
Reference
-
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–249. PMID: 33538338.
Article2. Stevens LA, McGrath MH, Druss RG, Kister SJ, Gump FE, Forde KA. The psychological impact of immediate breast reconstruction for women with early breast cancer. Plast Reconstr Surg. 1984; 73:619–628. PMID: 6709743.
Article3. Lam TC, Hsieh F, Boyages J. The effects of postmastectomy adjuvant radiotherapy on immediate two-stage prosthetic breast reconstruction: a systematic review. Plast Reconstr Surg. 2013; 132:511–518. PMID: 23676964.
Article4. Yip JM, Watson DI, Tiggemann M, Hsia S, Smallman AE, Dean NR. Determinants of breast reconstruction outcome: how important is volume symmetry? J Plast Reconstr Aesthet Surg. 2015; 68:679–685. PMID: 25731778.
Article5. Blondeel PN, Hijjawi J, Depypere H, Roche N, Van Landuyt K. Shaping the breast in aesthetic and reconstructive breast surgery: an easy three-step principle. Plast Reconstr Surg. 2009; 123:455–462. PMID: 19182601.
Article6. Hidalgo DA, Spector JA. Breast augmentation. Plast Reconstr Surg. 2014; 133:567e–583e.
Article7. Koch MC, Adamietz B, Jud SM, Fasching PA, Haeberle L, Karbacher S, et al. Breast volumetry using a three-dimensional surface assessment technique. Aesthetic Plast Surg. 2011; 35:847–855. PMID: 21487916.
Article8. Herold C, Reichelt A, Stieglitz LH, Dettmer S, Knobloch K, Lotz J, et al. MRI-based breast volumetry-evaluation of three different software solutions. J Digit Imaging. 2010; 23:603–610. PMID: 20066465.
Article9. Vidya R, Masià J, Cawthorn S, Berna G, Bozza F, Gardetto A, et al. Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: first multicenter European report on 100 cases. Breast J. 2017; 23:670–676. PMID: 28481477.
Article10. Burkhardt BR, Eades E. The effect of Biocell texturing and povidone-iodine irrigation on capsular contracture around saline-inflatable breast implants. Plast Reconstr Surg. 1995; 96:1317–1325. PMID: 7480228.
Article11. Kovacs , Eder M, Hollweck , Zimmermann A, Settles M, Schneider A, et al. Comparison between breast volume measurement using 3D surface imaging and classical techniques. Breast. 2007; 16:137–145. PMID: 17029808.
Article12. Yoo A, Minn KW, Jin US. Magnetic resonance imaging-based volumetric analysis and its relationship to actual breast weight. Arch Plast Surg. 2013; 40:203–208. PMID: 23730594.
Article13. Kovacs , Eder M, Hollweck , Zimmermann A, Settles M, Schneider A, et al. New aspects of breast volume measurement using 3-dimensional surface imaging. Ann Plast Surg. 2006; 57:602–610. PMID: 17122543.
Article14. Hudson DA. Factors determining shape and symmetry in immediate breast reconstruction. Ann Plast Surg. 2004; 52:15–21. PMID: 14676693.
Article15. Lee HY, Hong K, Kim EA. Measurement protocol of women’s nude breasts using a 3D scanning technique. Appl Ergon. 2004; 35:353–359. PMID: 15159200.
Article16. Kayar R, Civelek S, Cobanoglu M, Gungor O, Catal H, Emiroglu M. Five methods of breast volume measurement: a comparative study of measurements of specimen volume in 30 mastectomy cases. Breast Cancer (Auckl). 2011; 5:43–52. PMID: 21494401.
Article17. Bulstrode N, Bellamy E, Shrotria S. Breast volume assessment: comparing five different techniques. Breast. 2001; 10:117–123. PMID: 14965570.
Article18. Chae MP, Rozen WM, Spychal RT, Hunter-Smith DJ. Breast volumetric analysis for aesthetic planning in breast reconstruction: a literature review of techniques. Gland Surg. 2016; 5:212–226. PMID: 27047788.19. Kim JS, Bae K, Lee EJ, Bang M. Mammography with a fully automated breast volumetric software as a novel method for estimating the preoperative breast volume prior to mastectomy. Ann Surg Treat Res. 2021; 100:313–319. PMID: 34136427.
Article20. Rha EY, Choi IK, Yoo G. Accuracy of the method for estimating breast volume on three-dimensional simulated magnetic resonance imaging scans in breast reconstruction. Plast Reconstr Surg. 2014; 133:14–20.
Article21. Kim H, Mun GH, Wiraatmadja ES, Lim SY, Pyon JK, Oh KS, et al. Preoperative magnetic resonance imaging-based breast volumetry for immediate breast reconstruction. Aesthetic Plast Surg. 2015; 39:369–376. PMID: 25924697.
Article22. Fowler PA, Casey CE, Cameron GG, Foster MA, Knight CH. Cyclic changes in composition and volume of the breast during the menstrual cycle, measured by magnetic resonance imaging. Br J Obstet Gynaecol. 1990; 97:595–602. PMID: 2390502.
Article23. Konyer NB, Ramsay EA, Bronskill MJ, Plewes DB. Comparison of MR imaging breast coils. Radiology. 2002; 222:830–834. PMID: 11867809.
Article24. Tepper OM, Karp NS, Small K, Unger J, Rudolph L, Pritchard A, et al. Three-dimensional imaging provides valuable clinical data to aid in unilateral tissue expander-implant breast reconstruction. Breast J. 2008; 14:543–550. PMID: 19054001.
Article25. Szychta P, Raine C, Butterworth M, Stewart K, Witmanowski H, Zadrozny M, et al. Preoperative implant selection for two stage breast reconstruction with 3D imaging. Comput Biol Med. 2014; 44:136–143. PMID: 24377697.
Article26. Coltman CE, Steele JR, McGhee DE. Breast volume is affected by body mass index but not age. Ergonomics. 2017; 60:1576–1585. PMID: 28532249.
Article27. Lee S. Three-dimensional photography and its application to facial plastic surgery. Arch Facial Plast Surg. 2004; 6:410–414. PMID: 15545536.
Article28. Ciocca L, Scotti R. CAD-CAM generated ear cast by means of a laser scanner and rapid prototyping machine. J Prosthet Dent. 2004; 92:591–595. PMID: 15583570.
Article29. Avis NJ, Briggs NM, Kleinermann F, Hose DR, Brown BH, Edwards MH. Anatomical and physiological models for surgical simulation. Stud Health Technol Inform. 1999; 62:23–29. PMID: 10538363.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Magnetic Resonance Imaging-Based Volumetric Analysis and Its Relationship to Actual Breast Weight
- Mammography with a fully automated breast volumetric software as a novel method for estimating the preoperative breast volume prior to mastectomy
- Perioperative antibiotic prophylaxis in implant breast reconstruction
- Differences in complications and asymmetry in patients who did not receive a balancing procedure in two-stage and direct-to-implant breast reconstruction
- Patient Satisfaction with Implant Based Breast Reconstruction Associated with Implant Volume and Mastectomy Specimen Weight Ratio