J Korean Acad Oral Health.
2022 Sep;46(3):129-134. 10.11149/jkaoh.2022.46.3.129.
The inhibitory effects of Rheum palmatum extract on the growth of oral streptococci and biofilm formation
- Affiliations
-
- 1Department of Oral Microbiology, School of Dentistry, Pusan National University, Yangsan, Korea
- 2Oral Genomics Research Center, Pusan National University, Yangsan, Korea
- 3Education and Research Team for Life Science on Dentistry, Pusan National University, Yangsan, Korea
- KMID: 2534107
- DOI: http://doi.org/10.11149/jkaoh.2022.46.3.129
Abstract
Objectives
Oral streptococci play a significant role in the development of dental caries. Among them, Streptococcus mutans and Streptococcus sobrinus are the principal causative agents of dental caries. Rheum palmatum is a flowering plant of the family Polygonaceae with several known medicinal properties. However, its effects on oral streptococci have yet to be established. Therefore, we investigated the effects of Rheum palmatum for its potential use as an anticaries agent in inhibiting the growth of streptococci and preventing biofilm formation.
Methods
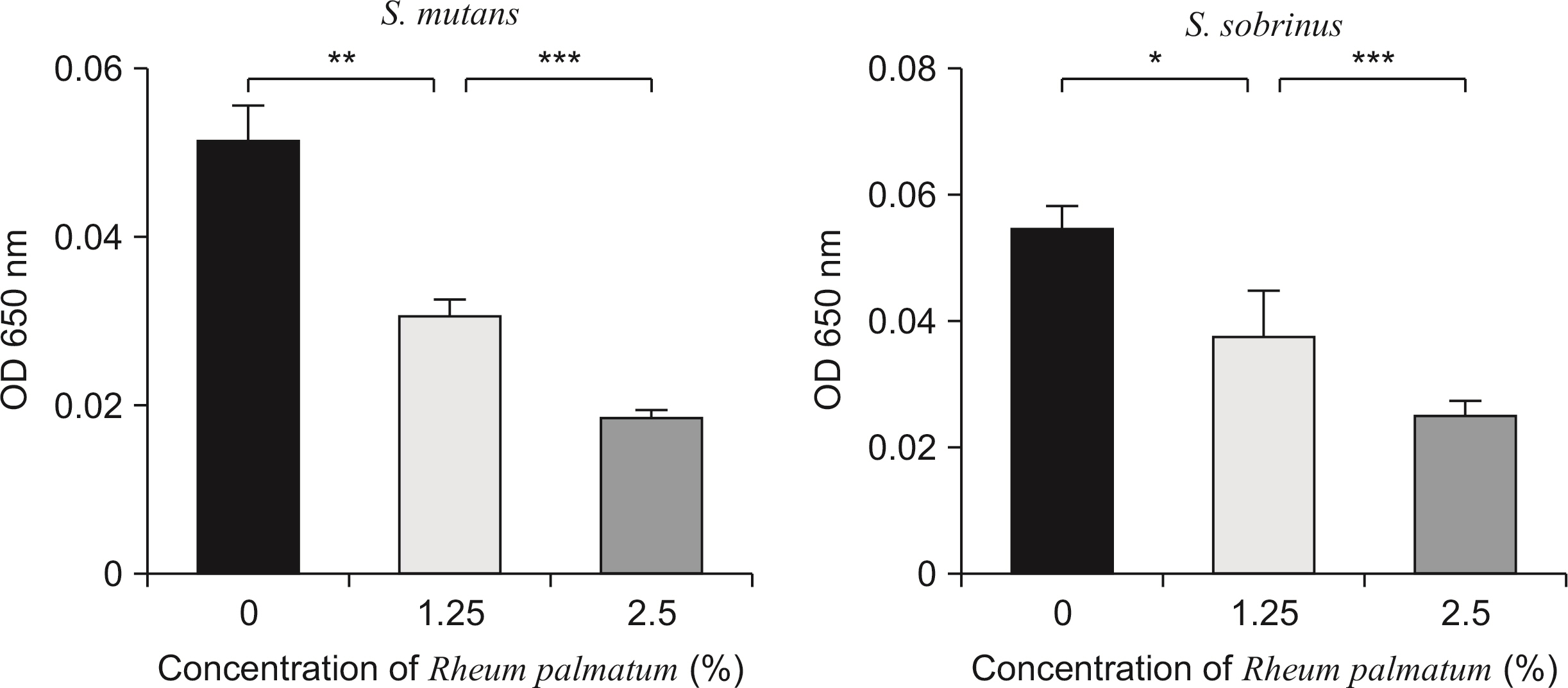
Rheum palmatum extract was diluted with sterile distilled water to obtain various extract concentrations. Several strains of oral bacteria, including S. mutans and S. sobrinus, were treated with the varying concentrations. The effects of the extract on bacterial growth was examined using the viable cell count method. Glucan synthesis was measured using a spectrophotometer at 650 nm optical density. Crystal violet staining was also carried out to observe the effect of the extract on biofilm formation.
Results
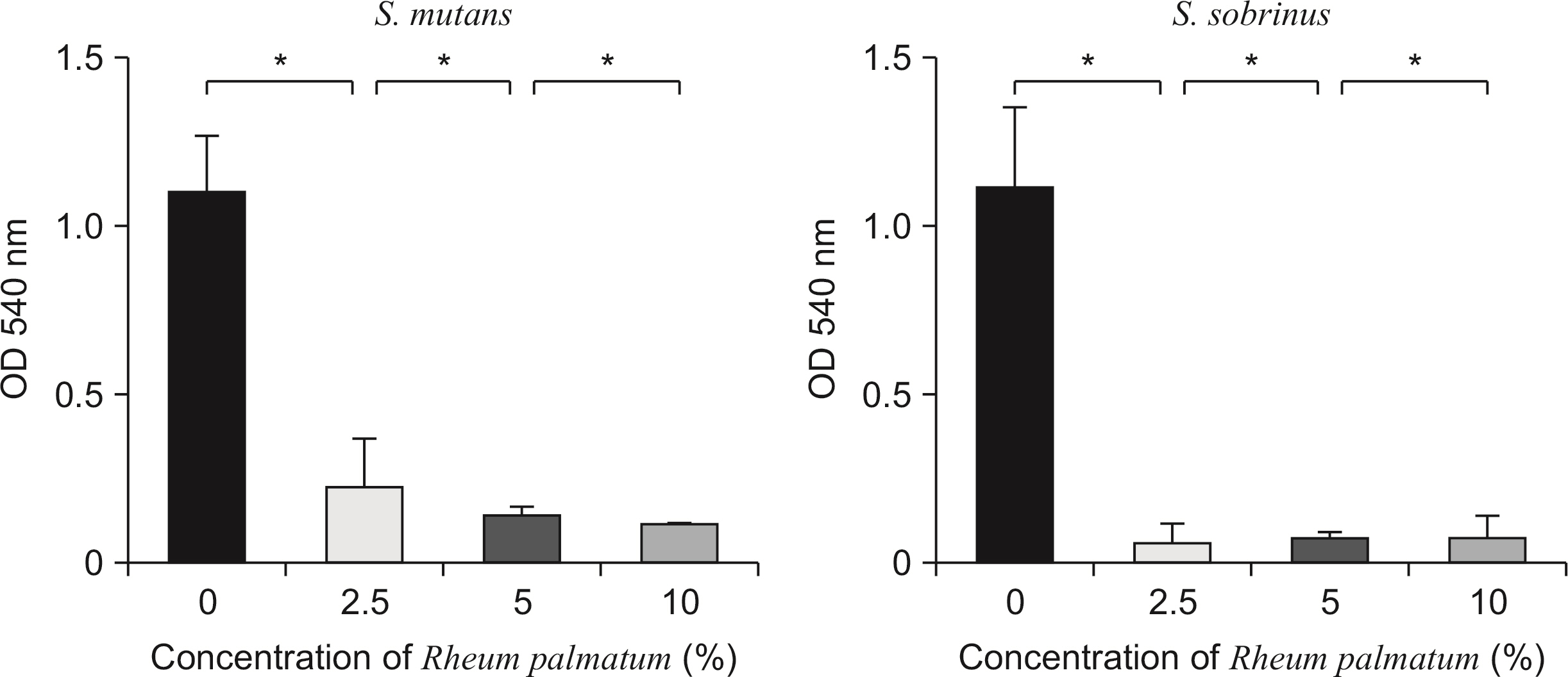
The growth of S. mutans and S. sobrinus was significantly inhibited by the Rheum palmatum solution at concentrations of 0.3% or more compared to the control group. The viable cell count results indicated that the number of bacterial colonies decreased 1.2-fold and 1.7-fold at concentrations of 1.25% and 2.5%, respectively, compared to the control group. Biofilm formation by S. mutans and S. sobrinus was suppressed more than 20-fold compared to the control group at extract concentrations of 1.25% or more.
Conclusions
The extract inhibited the growth of caries-causing bacteria, namely S. mutans and S. sobrinus. Furthermore, the extract inhibited the synthesis of glucan and biofilm formation by S. mutans and S. sobrinus. Therefore, this study suggests that the extract is a potential candidate as a therapeutic agent for controlling dental caries.
Figure
Reference
-
References
1. Categorical data by Korean Ministry of Health and Welfare [Internet]. Available from: http://Www.mohw.go.kr/react/jb/sjb1101vw.jspSEQ=89&MENU_ID=03320101&page=1&PAR_MENU_ID=03#.2. Berlutti F, Catizone A, Ricci G, Frioni A, Natalizi T, Valenti P, et al. 2010; Streptococcus mutans and streptococcus sobrinus are able to adhere and invade human gingival fibroblast cell line. Int J Immunopathol Pharmacol. 23:1253–1260. DOI: 10.1177/039463201002300430. PMID: 21244775.
Article3. Krasse Bo. 1988; Risco de cáriesGuia Prático para o Controle e Assessoramento. São Paulo Quintessence. 2:112.4. Taherkhani A, Ghonji F, Mazaheri A, Lohrasbi MP, Mohamadi Z, Khamverdi Z. 2021; Identification of potential glucosyltransferase inhibitors from cinnamic acid derivatives using molecular docking analysis: A bioinformatics study. Avicenna. J. Clin. Microb. Infec. 8:145–155. DOI: 10.34172/ajcmi.2021.27.
Article5. Seredin P, Goloshchapov D, Prutskij T, Ippolitov Y. 2015; Phase transformations in a human tooth tissue at the initial stage of caries. PLos One. 10:e0124008. DOI: 10.1371/journal.pone.0124008. PMID: 25901743. PMCID: PMC4406755.
Article6. Kang SY, An SY, Lee MW, Kwon SK, Lee DH, Jeon BH, et al. 2015; Effects of aconitum koreanum extract on the growth, acid production, adhesion and insoluble glucan synthesis of streptococcus mutans. J Physiol & Pathol Korean Med. 29:27–32. DOI: 10.15188/kjopp.2015.02.29.1.27.
Article7. Sakanaka S, Kim M, Taniguchi M, Yamamoto T. 1989; Antibacterial substances in japanese green tea extract against streptococcus mutans, a cariogenic bacterium. Agric Biol Chem. 53:2307–2311. DOI: 10.1271/bbb1961.53.2307.
Article8. Bae K. 1987; Seo WJ, Leem SH. The antibacterial activites of components isolated from the stem bark of magnolia obovota against a cariogenic bacterium, streptococcus mutans OMZ 176. Yakhak Hoeji. 36:36–39.9. Chen C, Lin C, Tsuneo N. 1989; Screening of taiwanese crude drugs for antibacterial activity against streptococcus mutans. J Ethnopharmacol. 27:285–295. DOI: 10.1016/0378-8741(89)90003-2. PMID: 2615434.
Article10. United States Department of Agriculture. 2014. Natural resources conservation service PLANTS database.11. Foust CM. 2014. Rhubarb: The wondrous drug. Princeton University Press.12. Aly MM, Gumgumjee NM. 2011; Antimicrobial efficacy of rheum palmatum, curcuma longa and alpinia officinarum extracts against some pathogenic microorganisms. Afr. J. Biotechnol. 10:12058–12063.13. Zheng T, Yu F, Yao J. 2013; Effect of rheum palmatum L. infusion on expression of NF-κB in the liver of rats with acute intrahepatic cholestasis. Zhejiang J Trad Chin Med. 48:380–381.14. Cao Y, Pu Z, Tang Y, Shen J, Chen Y, Kang A, et al. 2017; Advances in bio-active constituents, pharmacology and clinical applications of rhubarb. Chinese Medicine. 12:1–12. DOI: 10.1186/s13020-017-0158-5. PMID: 29299052. PMCID: PMC5745730.
Article15. Lee Kuo-Hsiung. 2002; Chinese and Related North American Herbs: Phyto-pharmacology and Therapeutic Values By Thomas SC Li.CRC Press, Boca Raton, FL.2002.xi 598 pp.15.5× 23 cm.$169.95. J. Med. Chem. 45:4585–4586. DOI: 10.1021/jm0203074.
Article16. Huang Q, Lu G, Shen H, Chung MC, Ong CN. 2007; Anti-cancer properties of anthraquinones from rhubarb. Med Res Rev. 27:609–630. DOI: 10.1002/med.20094. PMID: 17022020.
Article17. Shuangsuo D, Zhengguo Z, Yunru C, Xin Z, Baofeng W, Lichao Y, Yan'an C. 2006; Inhibition of the replication of hepatitis B virus in vitro by emodin. Med Sci Monit. 12:302–306. PMID: 16940925.18. Cai Y, Sun M, Xing J, Corke H. 2004; Antioxidant phenolic constituents in roots of rheum officinale and rubia cordifolia: Structure- radical scavenging activity relationships. J Agric Food Chem. 52:7884–7890. DOI: 10.1021/jf0489116. PMID: 15612771.
Article19. Xiang H, Zuo J, Guo F, Dong D. 2020; What we already know about rhubarb: A comprehensive review. 15:1–22. DOI: 10.1186/s13020-020-00370-6. PMID: 32863857. PMCID: PMC7448319. PMID: 5028bcee80284cbb91ce417a18f9ffff.
Article20. Tsai K, Hsien H, Chen L, Ting W, Yang Y, Kuo C, et al. 2013; Rhubarb inhibits hepatocellular carcinoma cell metastasis via GSK-3-β activation to enhance protein degradation and attenuate nuclear translocation of β-catenin. Food Chem. 138:278–285. DOI: 10.1016/j.foodchem.2012.10.038. PMID: 23265488.
Article21. Arokiyaraj S, Vincent S, Saravanan M, Lee Y, Oh YK, Kim KH. 2017; Green synthesis of silver nanoparticles using rheum palmatum root extract and their antibacterial activity against staphylococcus aureus and pseudomonas aeruginosa. Artif. Cells Nanomed. Biotechnol. 45:372–379. DOI: 10.3109/21691401.2016.1160403. PMID: 27023851.
Article22. McNeill K, Hamilton I. 2003; Acid tolerance response of biofilm cells of streptococcus mutans. FEMS Microbiol Lett. 221:25–30. DOI: 10.1016/S0378-1097(03)00164-2. PMID: 12694906.23. Bowen WH. 2002; Do we need to be concerned about dental caries in the coming millennium? Crit. rev. oral biol. med. 13:126–131. DOI: 10.1177/154411130201300203. PMID: 12097355.24. Mukasa H, Slade HD. 1973; Mechanism of adherence of streptococcus mutans to smooth surfaces. I. roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 8:555–562. DOI: 10.1128/iai.8.4.555-562.1973. PMID: 4582634. PMCID: PMC422891.
Article25. Namba T, Tsunezuka M, Hattori M. 1982; Dental caries prevention by traditional chinese medicines. Planta Med. 44:100–106. DOI: 10.1055/s-2007-971412. PMID: 7071194.
Article26. Ohnuki T, Takashio M, Okami Y, Umezawa H. 1981; The structure of a novel inhibitor of dextransucrase. Tetrahedron Lett. 22:1267–1270. DOI: 10.1016/S0040-4039(01)90292-8.
Article27. An B, Kwon I, Choi C. 1995; Inhibiory effect of novel flavan-3-ol isolated theobroma cacao L. husk on glucosyltransferase. Korean J. Food Sci. Technol. 27:92–96.28. Nakahara K, Kawabata S, Ono H, Ogura K, Tanaka T, Ooshima T, Hamada S. 1993; Inhibitory effect of oolong tea polyphenols on glycosyltransferases of mutans streptococci. Appl Environ Microbiol. 59:968–973. DOI: 10.1128/aem.59.4.968-973.1993. PMID: 8489234. PMCID: PMC202224.
Article29. Kim SY, Song Y, Lee HA, Na HS, Jung CJ, Bek GY, Chung J. 2020; Inhibitory effects of coptis chinensis extract on the growth and biofilm formation of streptococcus mutans and streptococcus sobrinus. Int J Oral Biol. 45:143–151. DOI: 10.11620/IJOB.2020.45.4.143.
Article30. Kim KJ, Park BI, An SY, Jeon BH, Choi NY, You YO, et al. 2016; Inhibitory effects of radix pulsatillae extract on insoluble glucan synthesis and adhesion of streptococcus mutans. J Physiol & Pathol Korean Med. 30:27–32. DOI: 10.15188/kjopp.2016.02.30.1.27.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibitory effects of Coptis chinensis extract on the growth and biofilm formation of Streptococcus mutans and Streptococcus sobrinus
- Inhibitory Effect of Korean Mugwort (Artemisia princeps Pamp.) Extract on Growth and Biofilm Formation of Streptococcus mutans
- Effect of Sub-minimal Inhibitory Concentration of Chlorhexidine on Biofilm Formation and Coaggregation of Early Colonizers, Streptococci and Actinomycetes
- Inhibitory Effect of Pentose on Biofilm Formation by Oral Bacteria
- Effect of Sub-Minimal Inhibitory Concentrations of Antibiotics on Biofilm Formation and Coaggregation of Streptococci and Actinomycetes