J Pathol Transl Med.
2022 Sep;56(5):239-248. 10.4132/jptm.2022.05.18.
Inflammation and tissue remodeling contribute to fibrogenesis in stricturing Crohn’s disease: image processing and analysis study
- Affiliations
-
- 1Department of Pathology, Albany Medical Center, Albany, NY, USA
- 2Division of Anatomic Pathology, Department of Pathology, Penn State Health Milton S. Hershey Medical Center, Hershey, PA, USA
- 3Division of Pathology, University of Texas Southwestern Medical Center, Dallas, TX, USA
- 4Department of Pathology, Johns Hopkins Hospital, Baltimore, MD, USA
- 5Albany Medical College, Albany, NY, USA
- 6GE Global Research, Niskayuna, NY, USA
- KMID: 2533700
- DOI: http://doi.org/10.4132/jptm.2022.05.18
Abstract
- Background
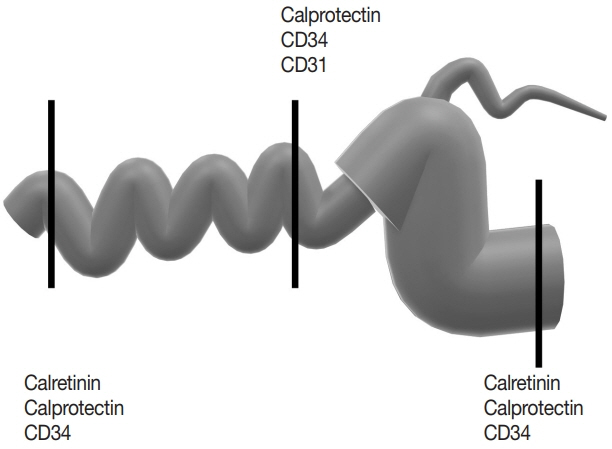
Inflammation and structural remodeling may contribute to fibrogenesis in Crohn’s disease (CD). We quantified the immunoexpression of calretinin, CD34, and calprotectin as a surrogate for mucosal innervation, telocytes (interstitial cells playing a role in networking), and inflammation, respectively, and correlated them with bowel alterations in stricturing CD.
Methods
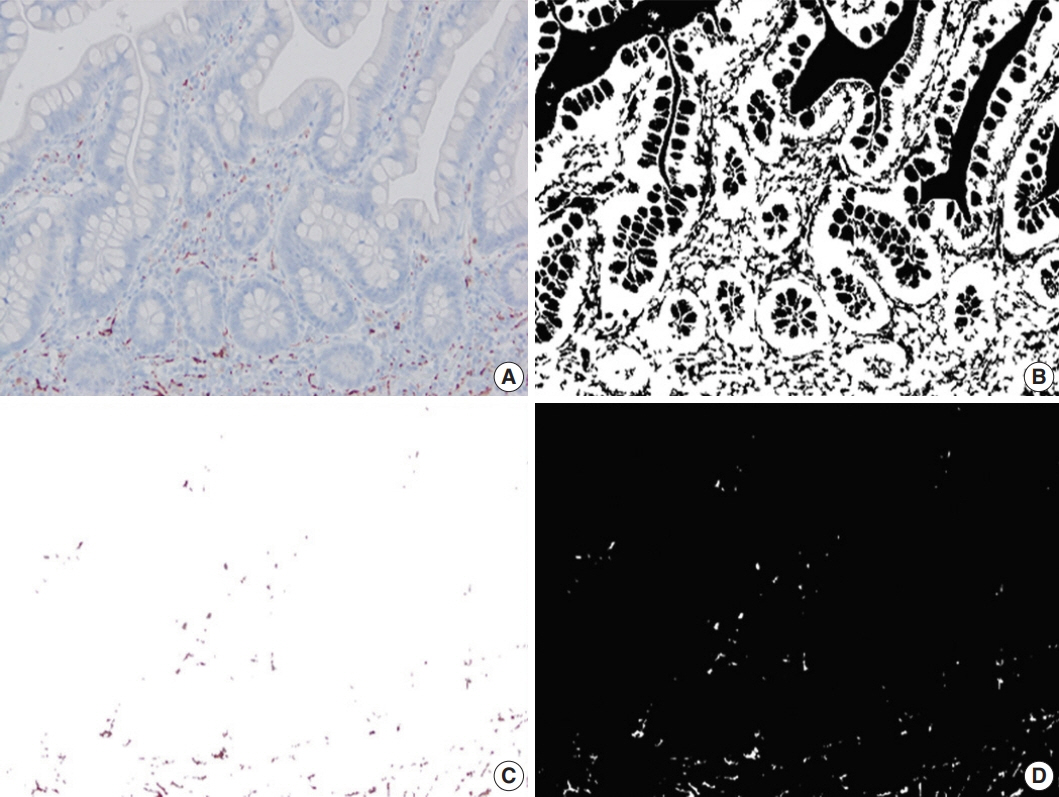
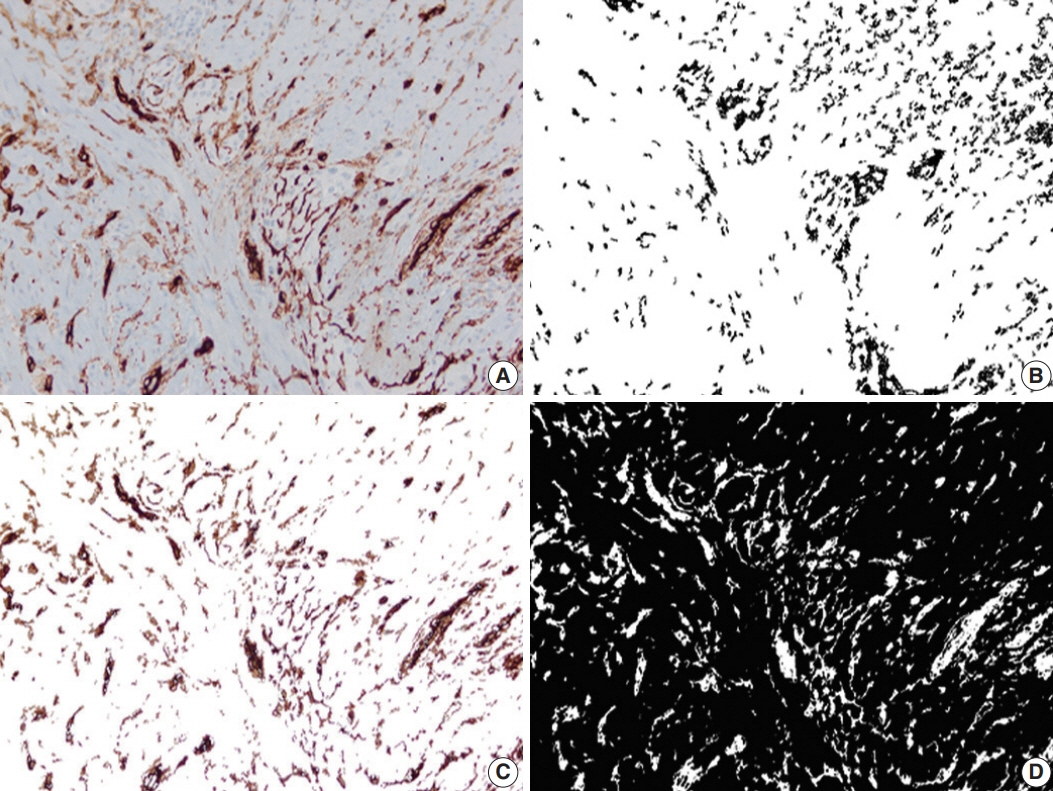
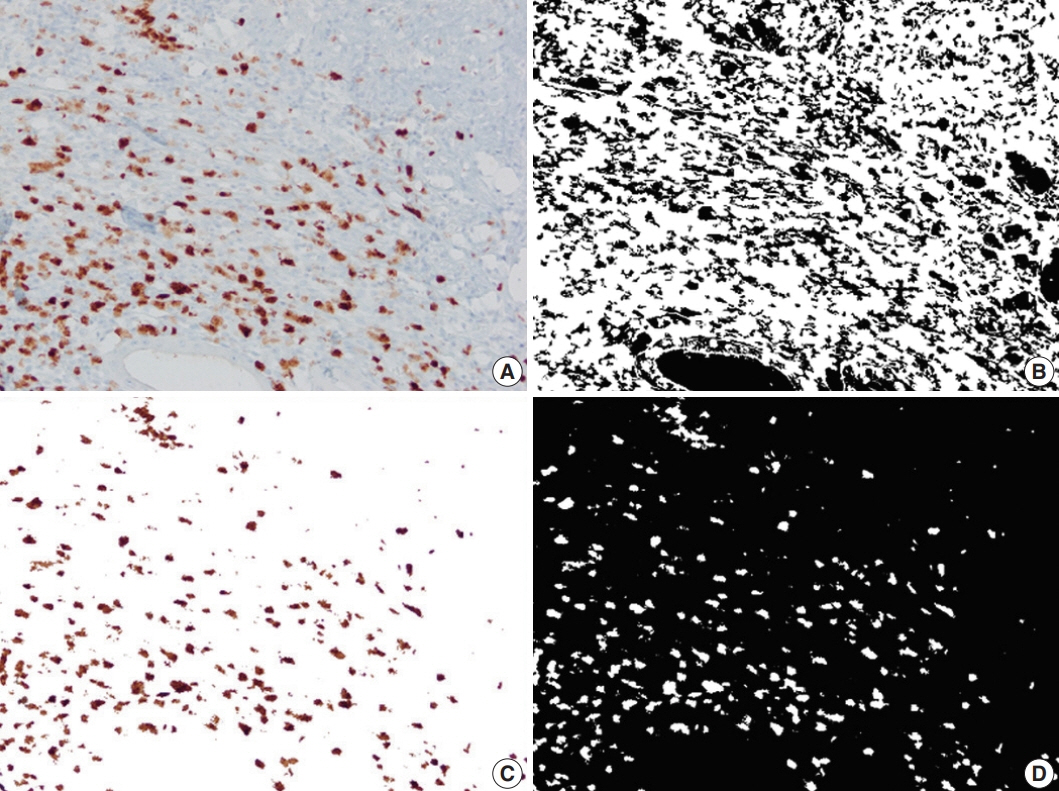
Primary resection specimens for ileal CD (n = 44, 31 stricturing CD, 13 inflammatory CD) were identified. Left-sided ulcerative colitis and trauma cases were used as controls. Proximal and distal margin and middle (diseased) sections were stained for calretinin, CD34, and calprotectin. Microscopic images were captured from the mucosa (calretinin), submucosa (calprotectin), and myenteric plexus (CD34), and the immunostaining was quantified using image processing and analysis. Bowel thickness at the corresponding sections were measured and correlated with the amount of immunoexpression.
Results
A total of 2,037 images were analyzed. In stricturing CD, submucosal alteration/thickening at the stricture site correlated with calprotectin staining and inversely correlated with calretinin staining at the proximal margin. Muscularis propria alteration/thickening at the stricture site correlated with mucosal calretinin staining at the proximal margin. Submucosal alteration/thickening at the proximal margin correlated with calretinin and CD34 staining at the proximal margin and inversely correlated with CD34 staining at the stricture site. Calretinin immunostaining at the distal margin was significantly higher in stricturing CD than the controls.
Conclusions
Inflammation and tissue remodeling appear to contribute to fibrogenesis in stricturing CD. Increased mucosal calretinin immunostaining distal to the diseased segment could be helpful in diagnosing CD in the right clinical context.
Keyword
Figure
Reference
-
References
1. Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn’s disease. Dis Mon. 2018; 64:20–57.
Article2. Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012; 142:46–54.
Article3. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006; 55:749–53.
Article4. Lee H, Westerhoff M, Shen B, Liu X. Clinical aspects of idiopathic inflammatory Bowel disease: a review for pathologists. Arch Pathol Lab Med. 2016; 140:413–28.
Article5. Ueno A, Jijon HB, Peng R, et al. Association of circulating fibrocytes with fibrostenotic small Bowel Crohn’s disease. Inflamm Bowel Dis. 2022; 28:246–58.
Article6. Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc. 2017; 92:1088–103.
Article7. Zeitz J, Fournier N, Labenz C, et al. Risk factors for the development of fistulae and stenoses in Crohn disease patients in the Swiss Inflammatory Bowel Disease Cohort. Inflamm Intest Dis. 2017; 1:172–81.
Article8. Chan WPW, Mourad F, Leong RW. Crohn’s disease associated strictures. J Gastroenterol Hepatol. 2018; 33:998–1008.
Article9. Rieder F, Fiocchi C, Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory Bowel diseases. Gastroenterology. 2017; 152:340–50.
Article10. Latella G, Sferra R, Speca S, Vetuschi A, Gaudio E. Can we prevent, reduce or reverse intestinal fibrosis in IBD? Eur Rev Med Pharmacol Sci. 2013; 17:1283–304.11. Gordon IO, Bettenworth D, Bokemeyer A, et al. Histopathology scoring systems of stenosis associated with small Bowel Crohn’s disease: a systematic review. Gastroenterology. 2020; 158:137–50.
Article12. Wu F, Chakravarti S. Differential expression of inflammatory and fibrogenic genes and their regulation by NF-kappaB inhibition in a mouse model of chronic colitis. J Immunol. 2007; 179:6988–7000.
Article13. Mathur R, Alam MM, Zhao XF, et al. Induction of autophagy in Cx3cr1(+) mononuclear cells limits IL-23/IL-22 axis-mediated intestinal fibrosis. Mucosal Immunol. 2019; 12:612–23.
Article14. Fukunaga S, Kuwaki K, Mitsuyama K, et al. Detection of calprotectin in inflammatory bowel disease: fecal and serum levels and immunohistochemical localization. Int J Mol Med. 2018; 41:107–18.
Article15. Kmeid M, Arker SH, Petchers A, et al. Appendiceal inflammation in colectomy is independently correlated with early pouchitis following ileal pouch anal anastomosis in ulcerative colitis and indeterminate colitis. Ann Diagn Pathol. 2021; 55:151838.
Article16. Milia AF, Ruffo M, Manetti M, et al. Telocytes in Crohn’s disease. J Cell Mol Med. 2013; 17:1525–36.
Article17. Wollheim FA. Telocytes, communicators in healthy stroma and relation to inflammation and fibrosis. Joint Bone Spine. 2016; 83:615–8.
Article18. Faussone-Pellegrini MS, Gherghiceanu M. Telocyte’s contacts. Semin Cell Dev Biol. 2016; 55:3–8.
Article19. Najjar S, Ahn S, Kasago I, et al. Image processing and analysis of mucosal calretinin staining to define the transition zone in Hirschsprung disease: a pilot study. Eur J Pediatr Surg. 2019; 29:179–87.
Article20. Cordeiro-Rudnisky F, Ahn S, Sheuka N, et al. Transition zone in total colonic aganglionosis and colorectal Hirschsprung’s disease shows a similar trend of mucosal innervation: image processing and analysis study. Pediatr Dev Pathol. 2020; 23:127–31.
Article21. Najjar S, Ahn S, Umrau K, et al. Increasing trend of calretinin-positive mucosal innervation from aganglionic zone toward transition zone in Hirschsprung's disease. Eur J Pediatr Surg. 2022; 32:191–7.
Article22. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012; 380:1590–605.
Article23. Strobach RS, Ross AH, Markin RS, Zetterman RK, Linder J. Neural patterns in inflammatory bowel disease: an immunohistochemical survey. Mod Pathol. 1990; 3:488–93.24. Belai A, Burnstock G. Distribution and colocalization of nitric oxide synthase and calretinin in myenteric neurons of developing, aging, and Crohn’s disease human small intestine. Dig Dis Sci. 1999; 44:1579–87.25. Chen W, Lu C, Hirota C, Iacucci M, Ghosh S, Gui X. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn’s fibrostenosing bowel strictures: a semiquantitative analysis by using a novel histological grading scheme. J Crohns Colitis. 2017; 11:92–104.
Article26. Guinard-Samuel V, Bonnard A, De Lagausie P, et al. Calretinin immunohistochemistry: a simple and efficient tool to diagnose Hirschsprung disease. Mod Pathol. 2009; 22:1379–84.
Article27. Popescu LM, Faussone-Pellegrini MS. Telocytes: a case of serendipity: the winding way from interstitial cells of cajal (ICC), via interstitial cajal-like cells (ICLC) to telocytes. J Cell Mol Med. 2010; 14:729–40.28. Irwin J, Ferguson E, Simms LA, Hanigan K, Carbonnel F, RadfordSmith G. A rolling phenotype in Crohn’s disease. PLoS One. 2017; 12:e0174954.
Article29. Ferrante M, de Hertogh G, Hlavaty T, et al. The value of myenteric plexitis to predict early postoperative Crohn’s disease recurrence. Gastroenterology. 2006; 130:1595–606.
Article30. Bressenot A, Chevaux JB, Williet N, et al. Submucosal plexitis as a predictor of postoperative surgical recurrence in Crohn’s disease. Inflamm Bowel Dis. 2013; 19:1654–61.
Article31. Tandon P, Malhi G, Abdali D, et al. Active margins, plexitis, and granulomas increase postoperative Crohn’s recurrence: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2021; 19:451–62.
Article32. Crespi M, Dulbecco P, De Ceglie A, Conio M. Strictures in Crohn’s disease: from pathophysiology to treatment. Dig Dis Sci. 2020; 65:1904–16.
Article33. Alfredsson J, Wick MJ. Mechanism of fibrosis and stricture formation in Crohn's disease. Scand J Immunol. 2020; 92:e12990.
Article34. Manetti M, Rosa I, Messerini L, Ibba-Manneschi L. Telocytes are reduced during fibrotic remodelling of the colonic wall in ulcerative colitis. J Cell Mol Med. 2015; 19:62–73.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rates of Early Surgery and Associated Risk Factors in Crohn's Disease
- Efficacy and tolerability of hyperbaric oxygen therapy in small bowel stricturing Crohn’s disease: a pilot study
- Design & Development of 3D Medical Image Processing System using VTK
- Diagnosis and treatment of Crohn's disease
- Simple post-processing of medical images