J Korean Neurosurg Soc.
2022 Sep;65(5):680-687. 10.3340/jkns.2021.0310.
Mild Traumatic Brain Injury and Subsequent Acute Pulmonary Inflammatory Response
- Affiliations
-
- 1Institute of New Frontier Research, Hallym University College of Medicine, Chuncheon, Korea
- 2Department of Neurosurgery, Hallym University College of Medicine, Chuncheon, Korea
- KMID: 2533030
- DOI: http://doi.org/10.3340/jkns.2021.0310
Abstract
Objective
: The influence of moderate-to-severe traumatic brain injury (TBI) on acute pulmonary injury is well established, but the association between acute pulmonary injury and mild TBI has not been well studied. Here, we evaluated the histological changes and fluctuations in inflammatory markers in the lungs to determine whether an acute pulmonary inflammatory response occurred after mild TBI.
Methods
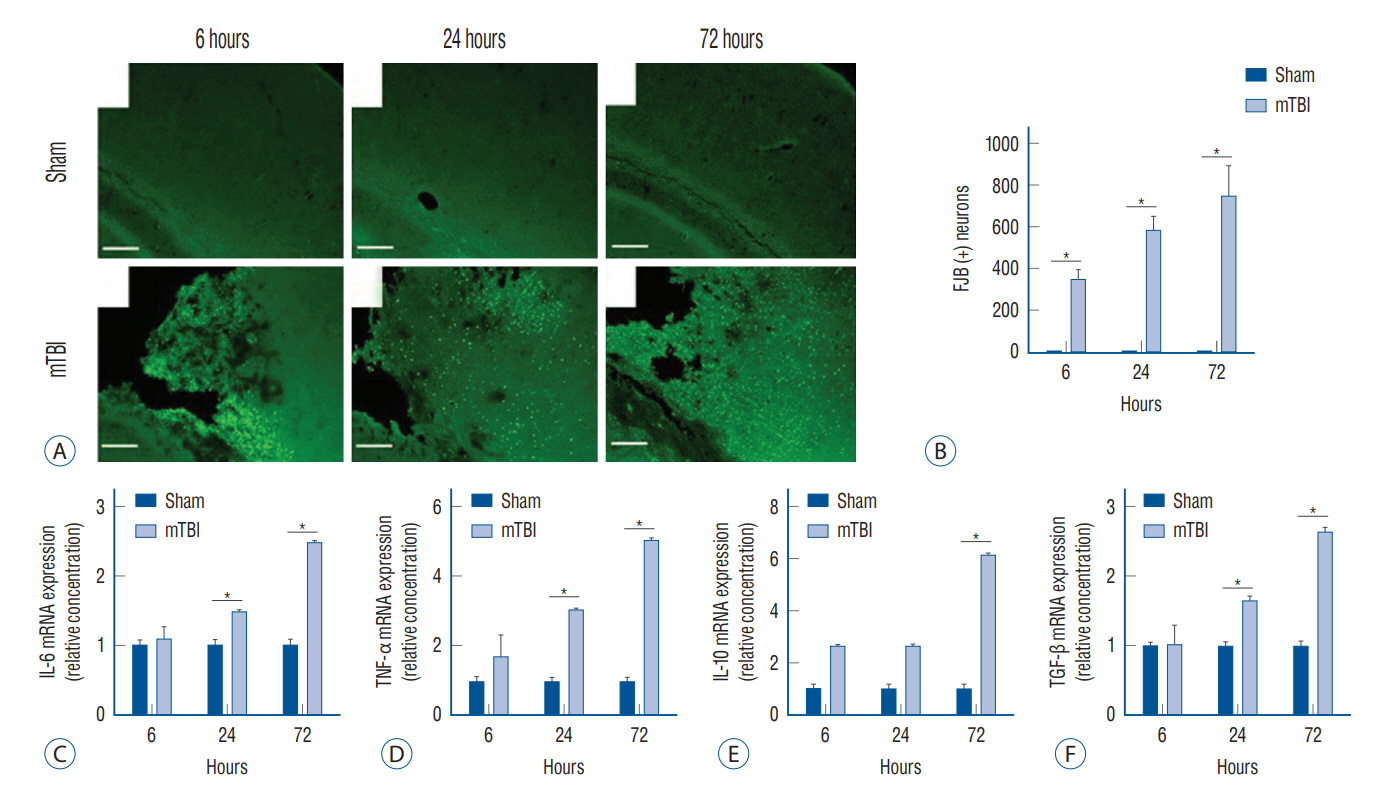
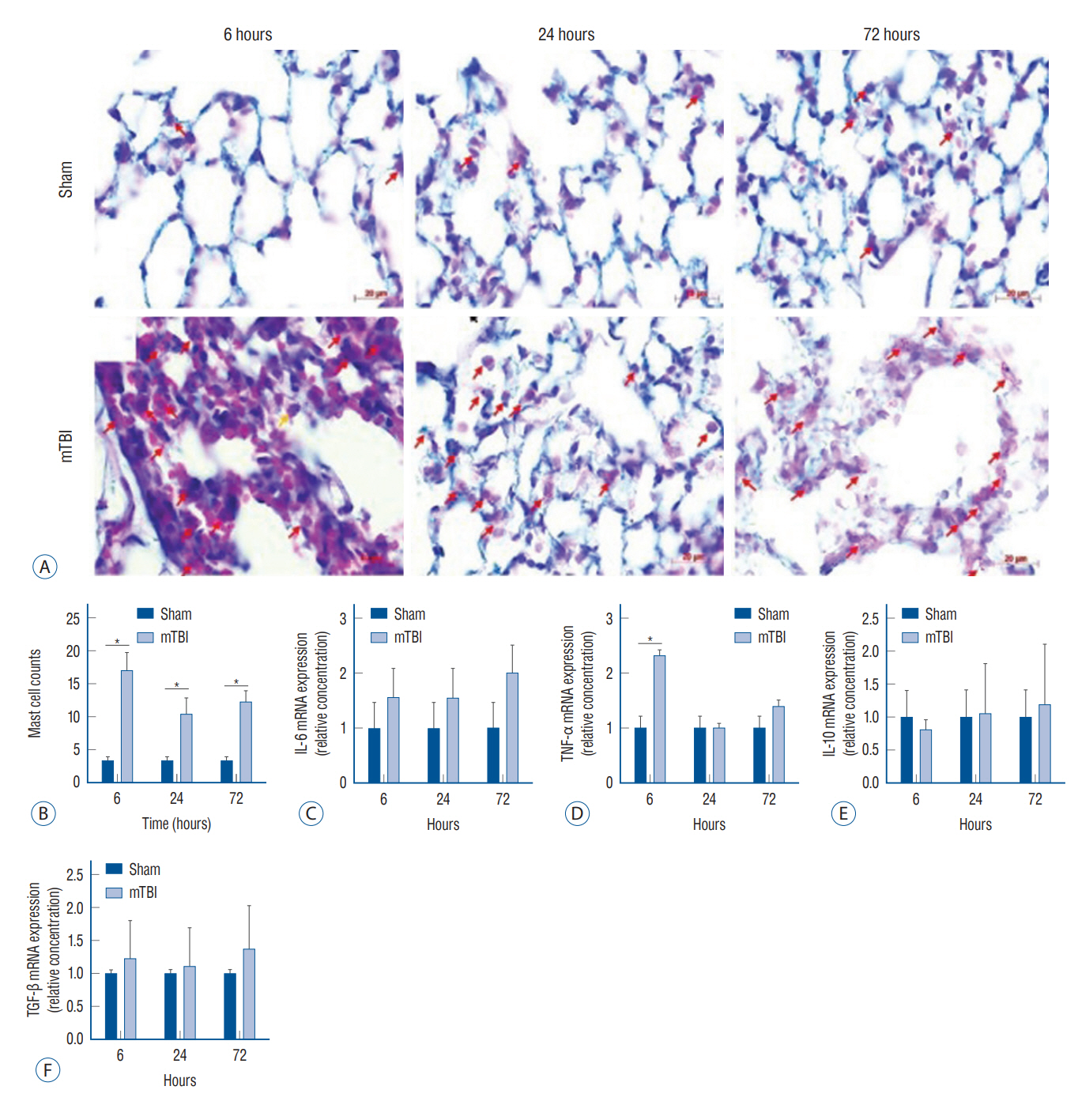
: Mouse models of mild TBI (n=24) were induced via open-head injuries using a stereotaxic impactor. The brain and lungs were examined 6, 24, and 72 hours after injury and compared to sham-operated controls (n=24). Fluoro-Jade B staining and Astra blue and hematoxylin staining were performed to assess cerebral neuronal degeneration and pulmonary histological architecture. Quantitative real-time polymerase chain reaction analysis was done to measure inflammatory cytokines.
Results
: Increased neuronal degeneration and the mRNA expression of interleukin (IL)-6, tumor necrosis factor (TNF)-α, IL-10, and transforming growth factor (TGF)-β were observed after mild TBI. The IL-6, TNF-α, and TGF-β levels in mice with mild TBI were significantly different compared to those of sham-operated mice 24 hours after injury, and this was more pronounced at 72 hours. Mild TBI induced acute pulmonary interstitial edema with cell infiltration and alveolar morphological changes. In particular, a significant infiltration of mast cells was observed. Among the inflammatory cytokines, TNF-α was significantly increased in the lungs at 6 hours, but there was no significant difference 24 and 72 hours after injury.
Conclusion
: Mild TBI induced acute pulmonary interstitial inflammation and alveolar structural changes, which are likely to worsen the patient’s prognosis.
Figure
Reference
-
References
1. Austin V, Ku JM, Miller AA, Vlahos R. Ischaemic stroke in mice induces lung inflammation but not acute lung injury. Sci Rep. 9:3622. 2019.2. Bae YH, Joo H, Bae J, Hyeon SJ, Her S, Ko E, et al. Brain injury induces HIF-1α-dependent transcriptional activation of LRRK2 that exacerbates brain damage. Cell Death Dis. 9:1125. 2018.3. Beller E, Reuter L, Kluge A, Preibisch C, Lindauer U, Bogdanov A, et al. Pilot study to assess visualization and therapy of inflammatory mechanisms after vessel reopening in a mouse stroke model. Sci Rep. 8:745. 2018.4. Brambrink AM, Dick WF. Neurogenic pulmonary edema. pathogenesis, clinical picture and therapy. Anaesthesist. 46:953–963. 1997.5. Cai C, Cao Z, Loughran PA, Kim S, Darwiche S, Korff S, et al. Mast cells play a critical role in the systemic inflammatory response and end-organ injury resulting from trauma. J Am Coll Surg. 213:604–615. 2011.6. Englert JA, Macias AA, Amador-Munoz D, Pinilla Vera M, Isabelle C, Guan J, et al. Isoflurane ameliorates acute lung injury by preserving epithelial tight junction integrity. Anesthesiology. 123:377–388. 2015.7. Graziottin A, Skaper SD, Fusco M. Mast cells in chronic inflammation, pelvic pain and depression in women. Gynecol Endocrinol. 30:472–477. 2014.8. Humphries DC, O’Neill S, Scholefield E, Dorward DA, Mackinnon AC, Rossi AG, et al. Cerebral concussion primes the lungs for subsequent neutrophil-mediated injury. Crit Care Med. 46:e937–e944. 2018.9. Kempuraj D, Selvakumar GP, Thangavel R, Ahmed ME, Zaheer S, Raikwar SP, et al. Mast cell activation in brain injury, stress, and posttraumatic stress disorder and alzheimer’s disease pathogenesis. Front Neurosci. 11:703. 2017.10. Kerr NA, De Rivero Vaccari JP, Abbassi S, Kaur H, Zambrano R, Wu S, et al. Traumatic brain injury-induced acute lung injury: evidence for activation and inhibition of a neural-respiratory-inflammasome axis. J Neurotrauma. 35:2067–2076. 2018.11. Lefevre-Dognin C, Cogne M, Perdrieau V, Granger A, Heslot C, Azouvi P. Definition and epidemiology of mild traumatic brain injury. Neurochirurgie. 67:218–221. 2021.12. Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 381:77–80. 1996.13. Nicolls MR, Laubach VE. Traumatic brain injury: lungs in a RAGE. Sci Transl Med. 6:252fs234. 2014.14. Prince JM, Levy RM, Yang R, Mollen KP, Fink MP, Vodovotz Y, et al. Toll-like receptor-4 signaling mediates hepatic injury and systemic inflammation in hemorrhagic shock. J Am Coll Surg. 202:407–417. 2006.15. Rincon F, Ghosh S, Dey S, Maltenfort M, Vibbert M, Urtecho J, et al. Impact of acute lung injury and acute respiratory distress syndrome after traumatic brain injury in the united States. Neurosurgery. 71:795–803. 2012.16. Rouzé A, Jaillette E, Nseir S. Relationship between microaspiration of gastric contents and ventilator-associated pneumonia. Ann Transl Med. 6:428. 2018.17. Vaickus M, Hsieh T, Kintsurashvili E, Kim J, Kirsch D, Kasotakis G, et al. Mild traumatic brain injury in mice beneficially alters lung NK1R and structural protein expression to enhance survival after pseudomonas aeruginosa infection. Am J Pathol. 189:295–307. 2019.18. Weber DJ, Gracon AS, Ripsch MS, Fisher AJ, Cheon BM, Pandya PH, et al. The HMGB1-RAGE axis mediates traumatic brain injury-induced pulmonary dysfunction in lung transplantation. Sci Transl Med. 6:252ra124. 2014.19. Youn DH, Tran NM, Kim BJ, Kim Y, Jeon JP, Yoo H. Shape effect of cerium oxide nanoparticles on mild traumatic brain injury. Sci Rep. 11:15571. 2021.20. Zhang J, Teng Z, Song Y, Hu M, Chen C. Inhibition of monoacylglycerol lipase prevents chronic traumatic encephalopathy-like neuropathology in a mouse model of repetitive mild closed head injury. J Cereb Blood Flow Metab. 35:443–453. 2015.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic History of Traumatic Axonal Injury in Patients with Cerebral Concussion and Mild Traumatic Brain Injury
- Animal Models of Traumatic Brain Injury
- The role of cardiac dysfunction and post-traumatic pulmonary embolism in brain-lung interactions following traumatic brain injury
- The Impact of Anxiety Symptoms on Cognitive Function in Patients with Mild Traumatic Brain Injury
- Controversies in Acute Care of Patients with Severe Traumatic Brain Injury