Ann Surg Treat Res.
2022 Sep;103(3):119-128. 10.4174/astr.2022.103.3.119.
Clinicopathological features and prognosis associated with breast cancer laterality: a nationwide study from the Korean Breast Cancer Society
- Affiliations
-
- 1Department of Surgery, Daejeon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Department of Surgery, Yeungnam University Medical Center, Yeungnam University College of Medicine, Daegu, Korea
- 3Department of Surgery, Jeonbuk National University Medical School, Jeonju, Korea
- 4Department of Surgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 5Department of Surgery, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 6Department of Surgery, Incheon St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 7Breast-Thyroid Center, Saegyaero Hospital, Busan, Korea
- KMID: 2532924
- DOI: http://doi.org/10.4174/astr.2022.103.3.119
Abstract
- Purpose
Although breast cancer is known to show a left predominance, the clinical characteristics and causes underlying this finding remain unclear. In addition, no related studies on breast cancer laterality have been conducted in patients with breast cancer in Korea. Therefore, we aimed to analyze differences in breast cancer laterality and the associated clinicopathological characteristics and prognosis among Korean patients with breast cancer.
Methods
We conducted a retrospective analysis using large-scale data on clinicopathological factors and prognosis differences related to breast cancer laterality from the Korean Breast Cancer Society Registration system. The left-to-right ratio (LRR) of breast cancer was calculated through binomial distribution, and factors related to breast cancer laterality were identified through logistic regression analysis. In addition, the differences in the survival rates for left and right breast cancers were analyzed using the Kaplan-Meier method and Cox proportional hazards model.
Results
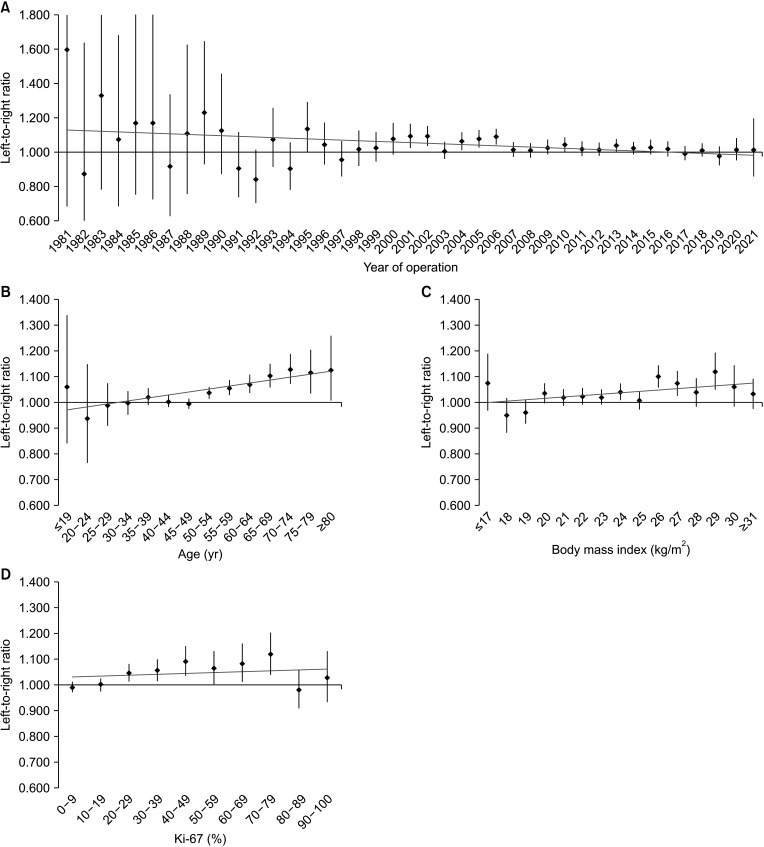
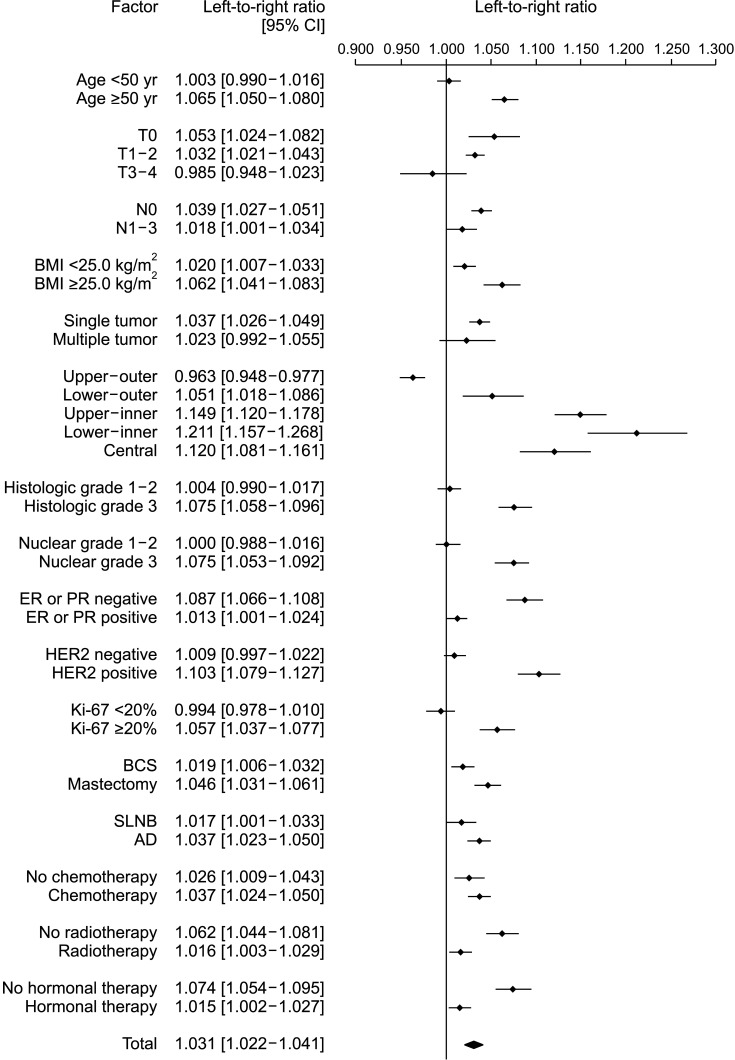
In 171,500 patients, the LRR was 1.031 (95% confidence interval, 1.022–1.041; P < 0.001). Multivariate analysis showed that the ratio of left breast cancer was related to age, body mass index (BMI), location, and human epidermal growth factor receptor 2 (HER2) status. The survival rate of patients with left and right breast cancers showed no significant difference.
Conclusion
A large-scale analysis revealed a left predominance in breast cancer laterality in Korean patients. Over time, this predominance gradually decreased. Age, BMI, location, and HER2 status affected breast cancer laterality. However, while left breast cancer showed relatively aggressive characteristics, it was not associated with a difference in the survival rate.
Keyword
Figure
Reference
-
1. Roychoudhuri R, Putcha V, Møller H. Cancer and laterality: a study of the five major paired organs (UK). Cancer Causes Control. 2006; 17:655–662. PMID: 16633912.
Article2. Busk T, Clemmesen J. The frequencies of left- and right-sided breast cancer. Br J Cancer. 1947; 1:345–351. PMID: 18906311.3. Perkins CI, Hotes J, Kohler BA, Howe HL. Association between breast cancer laterality and tumor location, United States, 1994-1998. Cancer Causes Control. 2004; 15:637–645. PMID: 15280621.4. Senie RT, Rosen PP, Lesser ML, Snyder RE, Schottenfeld D, Duthie K. Epidemiology of breast carcinoma II: factors related to the predominance of left-sided disease. Cancer. 1980; 46:1705–1713. PMID: 7417963.
Article5. Hsieh CC, Trichopoulos D. Breast size, handedness and breast cancer risk. Eur J Cancer. 1991; 27:131–135. PMID: 1827274.
Article6. Garfinkel L, Craig L, Seidman H. An appraisal of left and right breast cancer. J Natl Cancer Inst. 1959; 23:617–631. PMID: 13826569.7. Loughry CW, Sheffer DB, Price TE, Einsporn RL, Bartfai RG, Morek WM, et al. Breast volume measurement of 598 women using biostereometric analysis. Ann Plast Surg. 1989; 22:380–385. PMID: 2729845.
Article8. Kramer MA, Albrecht S, Miller RA. Handedness and the laterality of breast cancer in women. Nurs Res. 1985; 34:333–337. PMID: 2866491.
Article9. Ing R, Petrakis NL, Ho JH. Unilateral breast-feeding and breast cancer. Lancet. 1977; 2:124–127. PMID: 69205.
Article10. Kamby C, Andersen J, Ejlertsen B, Birkler NE, Rytter L, Zedeler K, et al. Pattern of spread and progression in relation to the characteristics of the primary tumour in human breast cancer. Acta Oncol. 1991; 30:301–308. PMID: 2036238.
Article11. Sughrue T, Brody JP. Breast tumor laterality in the United States depends upon the country of birth, but not race. PLoS One. 2014; 9:e103313. PMID: 25083708.
Article12. Rutter CE, Chagpar AB, Evans SB. Breast cancer laterality does not influence survival in a large modern cohort: implications for radiation-related cardiac mortality. Int J Radiat Oncol Biol Phys. 2014; 90:329–334. PMID: 25304793.
Article13. Kang SY, Lee SB, Kim YS, Kim Z, Kim HY, Kim HJ, et al. Breast cancer statistics in Korea, 2018. J Breast Cancer. 2021; 24:123–137. PMID: 33913273.
Article14. Ekbom A, Adami HO, Trichopoulos D, Lambe M, Hsieh CC, Pontén J. Epidemiologic correlates of breast cancer laterality (Sweden). Cancer Causes Control. 1994; 5:510–516. PMID: 7827237.
Article15. Wilting J, Hagedorn M. Left-right asymmetry in embryonic development and breast cancer: common molecular determinants? Curr Med Chem. 2011; 18:5519–5527. PMID: 22172062.
Article16. Caouette-Laberge L, Borsuk D. Congenital anomalies of the breast. Semin Plast Surg. 2013; 27:36–41. PMID: 24872738.17. Romanini MV, Calevo MG, Puliti A, Vaccari C, Valle M, Senes F, et al. Poland syndrome: a proposed classification system and perspectives on diagnosis and treatment. Semin Pediatr Surg. 2018; 27:189–199. PMID: 30078491.
Article18. Schmidt H. Supernumerary nipples: prevalence, size, sex and side predilection: a prospective clinical study. Eur J Pediatr. 1998; 157:821–823. PMID: 9809822.
Article19. Yotsumoto F, Oki E, Tokunaga E, Maehara Y, Kuroki M, Miyamoto S. HB-EGF orchestrates the complex signals involved in triple-negative and trastuzumab-resistant breast cancer. Int J Cancer. 2010; 127:2707–2717. PMID: 20499311.
Article20. Golding JP, Tsoni S, Dixon M, Yee KT, Partridge TA, Beauchamp JR, et al. Heparin-binding EGF-like growth factor shows transient left-right asymmetrical expression in mouse myotome pairs. Gene Expr Patterns. 2004; 5:3–9. PMID: 15533812.
Article21. Engin A. Obesity-associated breast cancer: analysis of risk factors. Adv Exp Med Biol. 2017; 960:571–606. PMID: 28585217.
Article22. Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017; 67:378–397. PMID: 28763097.
Article23. Roudabush FL, Pierce KL, Maudsley S, Khan KD, Luttrell LM. Transactivation of the EGF receptor mediates IGF-1-stimulated shc phosphorylation and ERK1/2 activation in COS-7 cells. J Biol Chem. 2000; 275:22583–22589. PMID: 10807918.
Article24. Mulligan C, Rochford J, Denyer G, Stephens R, Yeo G, Freeman T, et al. Microarray analysis of insulin and insulin-like growth factor-1 (IGF-1) receptor signaling reveals the selective up-regulation of the mitogen heparin-binding EGF-like growth factor by IGF-1. J Biol Chem. 2002; 277:42480–42487. PMID: 12213819.
Article25. Amer MH. Genetic factors and breast cancer laterality. Cancer Manag Res. 2014; 6:191–203. PMID: 24790468.
Article26. Veltmaat JM, Ramsdell AF, Sterneck E. Positional variations in mammary gland development and cancer. J Mammary Gland Biol Neoplasia. 2013; 18:179–188. PMID: 23666389.
Article27. Robichaux JP, Hallett RM, Fuseler JW, Hassell JA, Ramsdell AF. Mammary glands exhibit molecular laterality and undergo left-right asymmetric ductal epithelial growth in MMTV-cNeu mice. Oncogene. 2015; 34:2003–2010. PMID: 24909172.
Article28. Scutt D, Lancaster GA, Manning JT. Breast asymmetry and predisposition to breast cancer. Breast Cancer Res. 2006; 8:R14. PMID: 16563179.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Surgery of Breast Cancer during the Last 5 Years: More Sophisticated and Specialized?
- The Highligts of 28th Annual Meeting of San Antonio Breast Cancer Symposium
- Mono- and Combination Chemotherapy for Metastatic Breast Cancer: An Increamental Step Forward
- Aromtase Inhibitors in Breast Cancer
- Diagnosis and Treatment of HER2-Positive Breast Cancer