Int J Stem Cells.
2022 Aug;15(3):291-300. 10.15283/ijsc21116.
Xenogeneic Humoral Immune Responses to Human Mesenchymal Stem Cells in Mice
- Affiliations
-
- 1Department of Anatomy and Cell Biology, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Biomedical Sciences, Seoul National University College of Medicine, Seoul, Korea
- 3BK21FOURs Biomedical Science Project, Seoul National University College of Medicine, Seoul, Korea
- 4Medical Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 5Wide River Institute of Immunology, Seoul National University, Hongcheon, Korea
- 6Department of Biomedical Laboratory Science, College of Health Science, Cheongju University, Cheongju, Korea
- KMID: 2532402
- DOI: http://doi.org/10.15283/ijsc21116
Abstract
- Background and Objectives
Many preclinical studies have been conducted using animal disease models to determine the effectiveness of human mesenchymal stem cells (hMSCs) for treating immune and inflammatory diseases based on the belief that hMSCs are not immunogenic across species. However, several researchers have suggested xenogeneic immune responses to hMSCs in animals, still without detailed features. This study aimed to investigate a xenogeneic humoral immune response to hMSCs in mice in detail.
Methods and Results
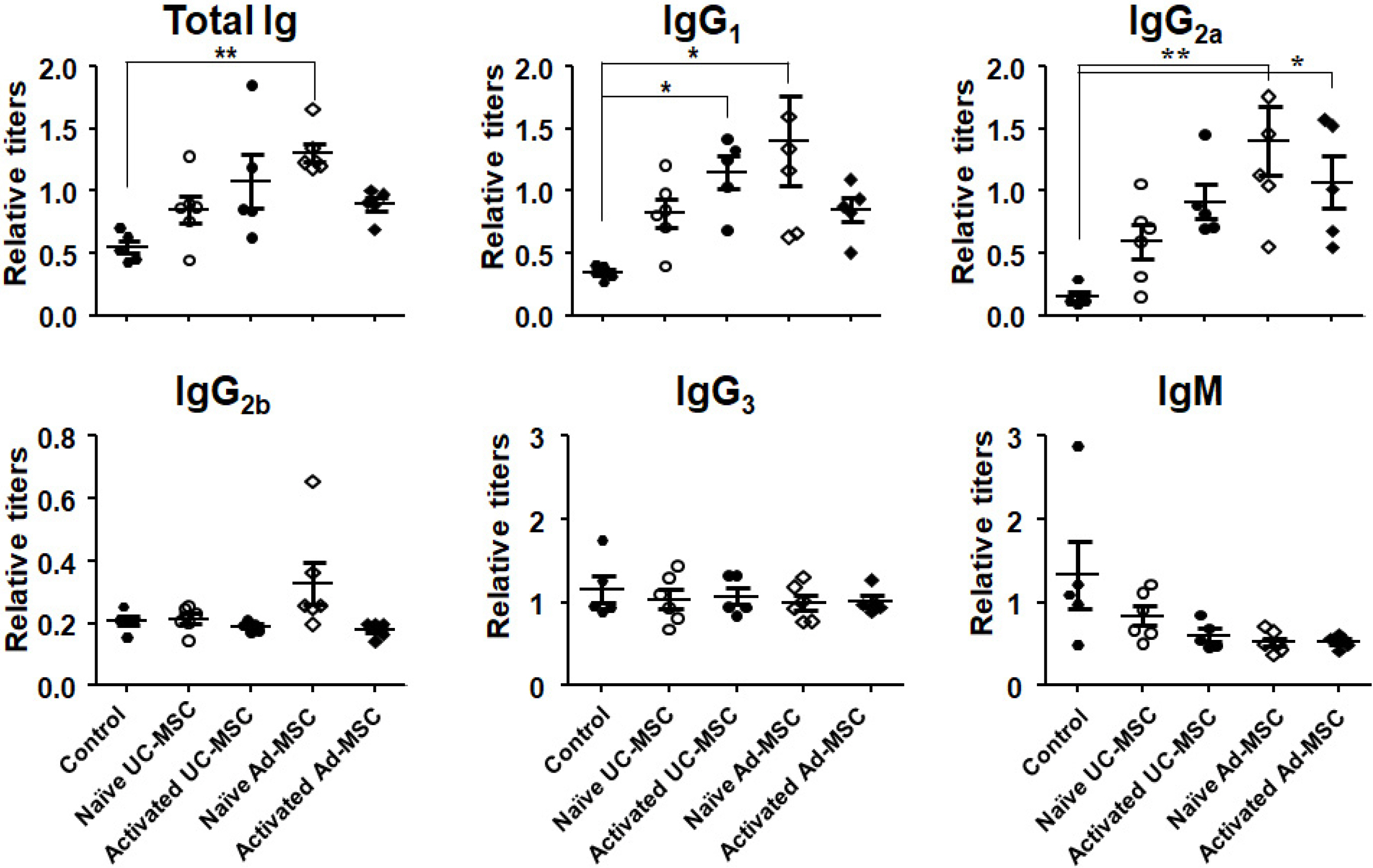
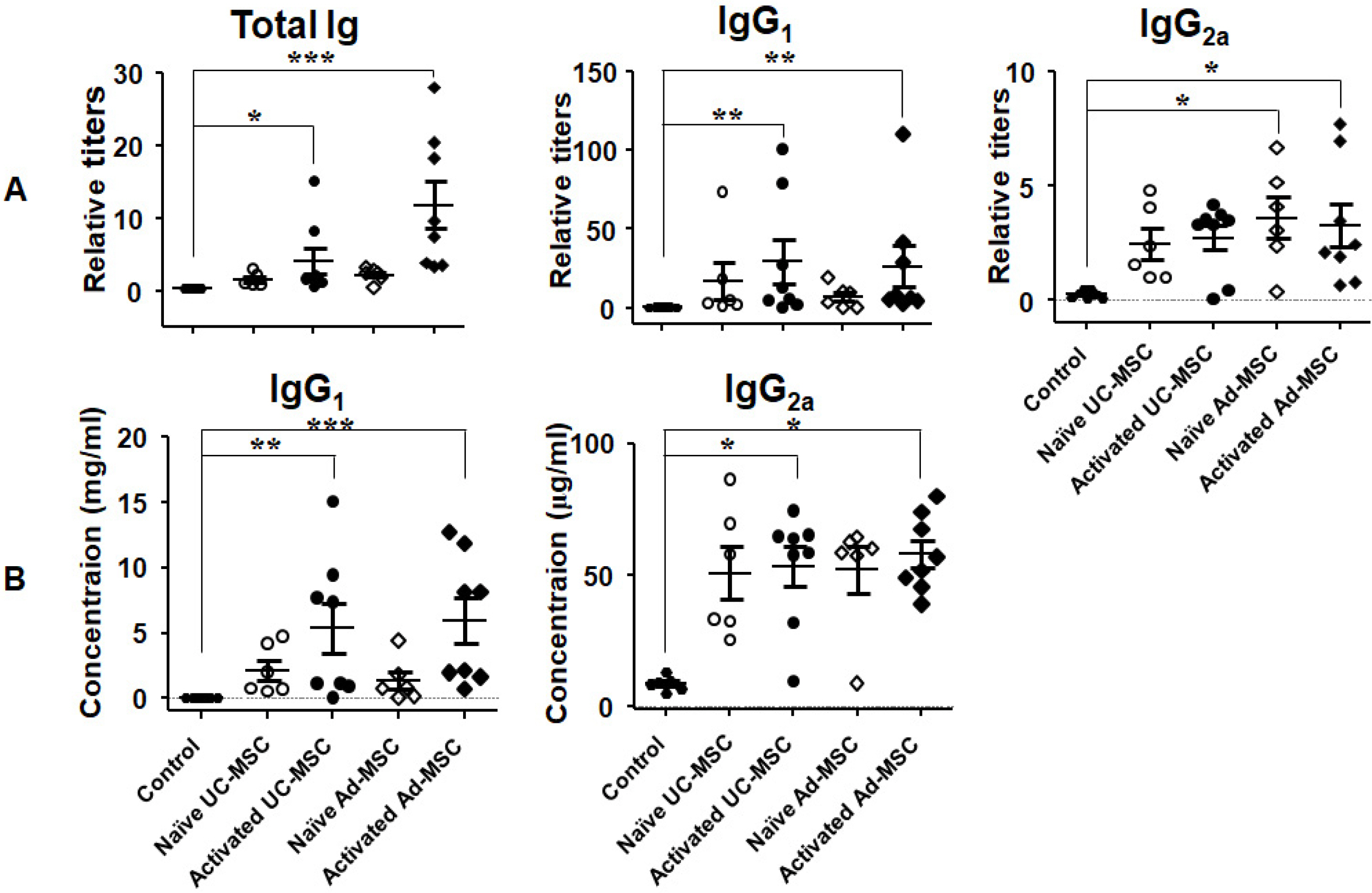
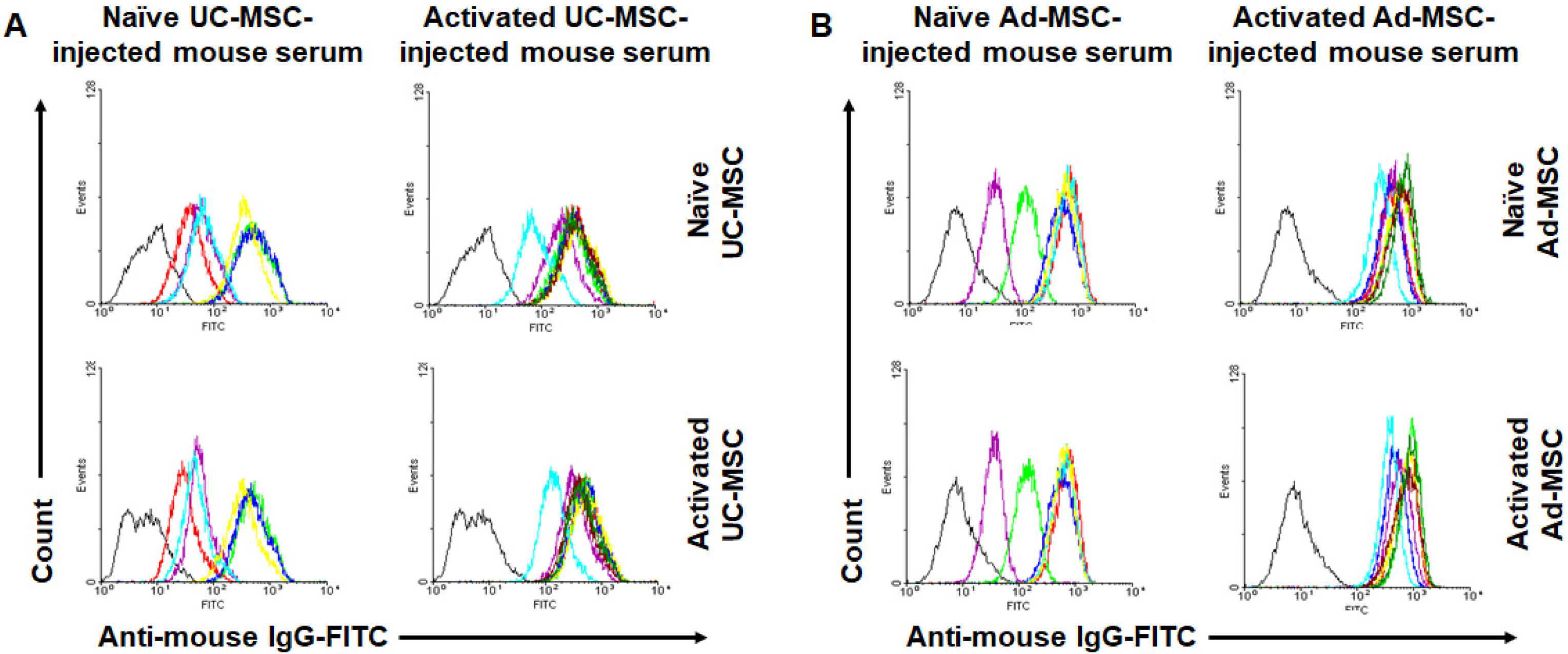
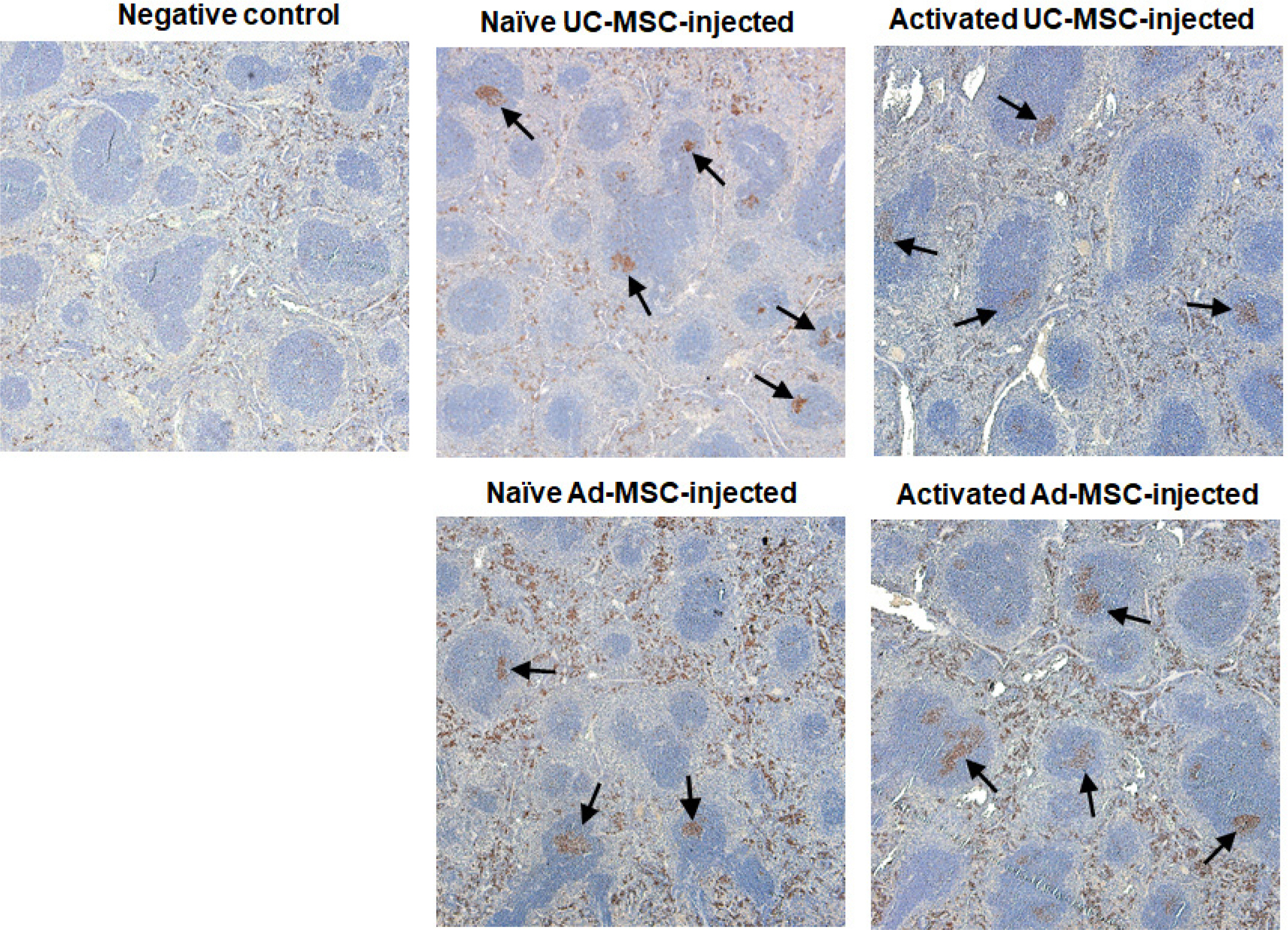
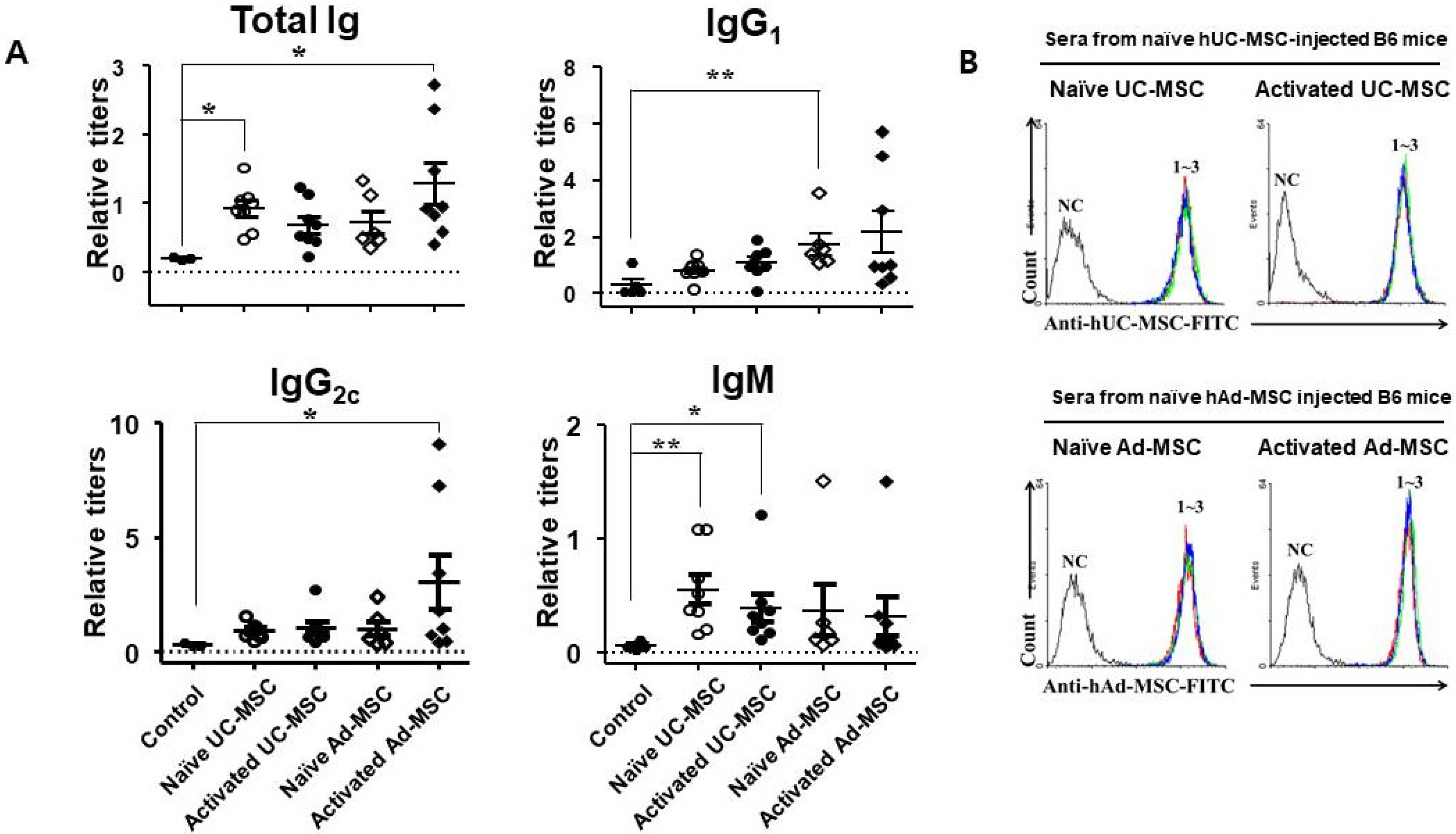
Balb/c mice were intraperitoneally injected with adipose tissue-derived or Wharton’s jelly-derived hMSCs. Sera from these mice were titrated for each isotype. To confirm specificity of the antibodies, hMSCs were stained with the sera and subjected to a flow cytometic analysis. Spleens were immunostained for proliferating cell nuclear antigen to verify the germinal center formation. Additionally, splenocytes were subjected to a flow cytometric analysis for surface markers including GL-7, B220, CD4, CD8, CD44, and CD62L. Similar experiments were repeated in C57BL/6 mice. The results showed increased IgG 1 and IgG 2a titers in the sera from Balb/c mice injected with hMSCs, and the titers were much higher in the secondary sera than in the primary sera. These antibodies were specifically stained the hMSCs. Germinal centers were observed in the spleen, and flow cytometric analysis of the splenocytes showed higher frequencies of centroblasts (B220 + GL7 + ) and memory T cells (CD62L + CD44 + ) both in CD4 + and CD8 + subsets. Similar results were obtained for C57BL/6 mice.
Conclusions
hMSCs induced a humoral immune response in mice, with characters of T cell-dependent immunity
Keyword
Figure
Reference
-
References
1. To K, Zhang B, Romain K, Mak C, Khan W. 2019; Synovium-derived mesenchymal stem cell transplantation in cartilage regeneration: a PRISMA review of in vivo studies. Front Bioeng Biotechnol. 7:314. DOI: 10.3389/fbioe.2019.00314. PMID: 31803726. PMCID: PMC6873960. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85076040986&origin=inward.2. Ansari AS, Yazid MD, Sainik NQAV, Razali RA, Saim AB, Idrus RBH. 2018; Osteogenic induction of Wharton's jelly-de-rived mesenchymal stem cell for bone regeneration: a systematic review. Stem Cells Int. 2018:2406462. DOI: 10.1155/2018/2406462. PMID: 30534156. PMCID: PMC6252214. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85062864268&origin=inward.3. Hu C, Zhao L, Li L. 2019; Current understanding of adipose-derived mesenchymal stem cell-based therapies in liver diseases. Stem Cell Res Ther. 10:199. DOI: 10.1186/s13287-019-1310-1. PMID: 31287024. PMCID: PMC6613269. PMID: b0bb20371bbc401398e771d293aea5f9. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85068928532&origin=inward.4. Cofano F, Boido M, Monticelli M, Zenga F, Ducati A, Vercelli A, Garbossa D. 2019; Mesenchymal stem cells for spinal cord injury: current options, limitations, and future of cell therapy. Int J Mol Sci. 20:2698. DOI: 10.3390/ijms20112698. PMID: 31159345. PMCID: PMC6600381. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85067174633&origin=inward.5. Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. 2004; Treatment of severe acute graft-versus-host disease with third party haploiden-tical mesenchymal stem cells. Lancet. 363:1439–1441. DOI: 10.1016/S0140-6736(04)16104-7. PMID: 15121408. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=2342482526&origin=inward.6. Álvaro-Gracia JM, Jover JA, García-Vicuña R, Carreño L, Alonso A, Marsal S, Blanco F, Martínez-Taboada VM, Taylor P, Martín-Martín C, DelaRosa O, Tagarro I, Díaz-González F. 2017; Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis. 76:196–202. DOI: 10.1136/annrheumdis-2015-208918. PMID: 27269294. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84973304693&origin=inward.7. Zhang J, Lv S, Liu X, Song B, Shi L. 2018; Umbilical cord mesenchymal stem cell treatment for Crohn's disease: a randomized controlled clinical trial. Gut Liver. 12:73–78. DOI: 10.5009/gnl17035. PMID: 28873511. PMCID: PMC5753687. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85040510160&origin=inward.8. Schepers K, Fibbe WE. 2016; Unraveling mechanisms of mesenchymal stromal cell-mediated immunomodulation through patient monitoring and product characterization. Ann N Y Acad Sci. 1370:15–23. DOI: 10.1111/nyas.12984. PMID: 26713608. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84976617562&origin=inward.9. Prockop DJ, Oh JY, Lee RH. 2017; Data against a common assumption: xenogeneic mouse models can be used to assay suppression of immunity by human MSCs. Mol Ther. 25:1748–1756. DOI: 10.1016/j.ymthe.2017.06.004. PMID: 28647464. PMCID: PMC5542802. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85021175505&origin=inward.10. Buja LM, Vela D. 2010; Immunologic and inflammatory reactions to exogenous stem cells implications for experimen-tal studies and clinical trials for myocardial repair. J Am Coll Cardiol. 56:1693–1700. DOI: 10.1016/j.jacc.2010.06.041. PMID: 21070919. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=78349245568&origin=inward.11. Pigott JH, Ishihara A, Wellman ML, Russell DS, Bertone AL. 2013; Investigation of the immune response to autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet Immunol Immuno-pathol. 156:99–106. DOI: 10.1016/j.vetimm.2013.09.003. PMID: 24094688. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84887050176&origin=inward.12. Grinnemo KH, Månsson A, Dellgren G, Klingberg D, Wardell E, Drvota V, Tammik C, Holgersson J, Ringdén O, Sylvén C, Le Blanc K. 2004; Xenoreactivity and engraftment of human mesenchymal stem cells transplanted into infarcted rat myocardium. J Thorac Cardiovasc Surg. 127:1293–1300. DOI: 10.1016/j.jtcvs.2003.07.037. PMID: 15115985. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=2342427573&origin=inward.13. Hwang JW, Lee NK, Yang JH, Son HJ, Bang SI, Chang JW, Na DL. 2020; A comparison of immune responses exerted following syngeneic, allogeneic, and xenogeneic transplantation of mesenchymal stem cells into the mouse brain. Int J Mol Sci. 21:3052. DOI: 10.3390/ijms21093052. PMID: 32357509. PMCID: PMC7246520. PMID: dffeb7a1acc34da481e4611252b44e91. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85084062470&origin=inward.14. Choi EW, Lee HW, Shin IS, Park JH, Yun TW, Youn HY, Kim SJ. 2016; Comparative efficacies of long-term serial transplantation of syngeneic, allogeneic, xenogeneic, or CTLA4Ig-overproducing xenogeneic adipose tissue-derived mesenchymal stem cells on murine systemic lupus erythematosus. Cell Transplant. 25:1193–1206. DOI: 10.3727/096368915X689442. PMID: 26377835. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84973168502&origin=inward.15. Kim JH, Lee YT, Hong JM, Hwang YI. 2013; Suppression of in vitro murine T cell proliferation by human adipose tissue-derived mesenchymal stem cells is dependent mainly on cyclooxygenase-2 expression. Anat Cell Biol. 46:262–271. DOI: 10.5115/acb.2013.46.4.262. PMID: 24386599. PMCID: PMC3875844.16. Jo CH, Kim OS, Park EY, Kim BJ, Lee JH, Kang SB, Lee JH, Han HS, Rhee SH, Yoon KS. 2008; Fetal mesenchymal stem cells derived from human umbilical cord sustain primitive characteristics during extensive expansion. Cell Tissue Res. 334:423–433. DOI: 10.1007/s00441-008-0696-3. PMID: 18941782. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=59449088430&origin=inward.17. Kim JH, Lee YT, Oh K, Cho J, Lee DS, Hwang YI. 2014; Paradoxical effects of human adipose tissue-derived mesenchymal stem cells on progression of experimental arthritis in SKG mice. Cell Immunol. 292:94–101. DOI: 10.1016/j.cellimm.2014.10.005. PMID: 25460084. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84911395004&origin=inward.18. Kim JH, Jo CH, Kim HR, Hwang YI. 2018; Comparison of immunological characteristics of mesenchymal stem cells from the periodontal ligament, umbilical cord, and adipose tissue. Stem Cells Int. 2018:8429042. DOI: 10.1155/2018/8429042. PMID: 29760736. PMCID: PMC5901833. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85056141943&origin=inward.19. Martin RM, Brady JL, Lew AM. 1998; The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 212:187–192. DOI: 10.1016/S0022-1759(98)00015-5. PMID: 9672206. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0032520727&origin=inward.20. Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. 1988; Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 334:255–258. DOI: 10.1038/334255a0. PMID: 2456466. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0023733838&origin=inward.21. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. 2000; M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 164:6166–6173. DOI: 10.4049/jimmunol.164.12.6166. PMID: 10843666. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0034659784&origin=inward.22. Jang IK, Jung HJ, Noh OK, Lee DH, Lee KC, Park JE. 2018; B7‑H1‑mediated immunosuppressive properties in human mesenchymal stem cells are mediated by STAT‑1 and not PI3K/Akt signaling. Mol Med Rep. 18:1842–1848. DOI: 10.3892/mmr.2018.9102. PMID: 29901104. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85049589022&origin=inward.23. Wang Q, Yang Q, Wang Z, Tong H, Ma L, Zhang Y, Shan F, Meng Y, Yuan Z. 2016; Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton's jelly as sources of cell immunomo-dulatory therapy. Hum Vaccin Immunother. 12:85–96. DOI: 10.1080/21645515.2015.1030549. PMID: 26186552. PMCID: PMC4962749. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84961827503&origin=inward.24. Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. 2006; Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 177:2080–2087. DOI: 10.4049/jimmunol.177.4.2080. PMID: 16887966. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33746898242&origin=inward.25. Chiesa S, Morbelli S, Morando S, Massollo M, Marini C, Bertoni A, Frassoni F, Bartolomé ST, Sambuceti G, Traggiai E, Uccelli A. 2011; Mesenchymal stem cells impair in vivo T-cell priming by dendritic cells. Proc Natl Acad Sci U S A. 108:17384–17389. DOI: 10.1073/pnas.1103650108. PMID: 21960443. PMCID: PMC3198360. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=80054809361&origin=inward.26. Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. 2006; Human mesenchymal stem cells modulate B-cell functions. Blood. 107:367–372. DOI: 10.1182/blood-2005-07-2657. PMID: 16141348. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=30144440925&origin=inward.27. Franquesa M, Mensah FK, Huizinga R, Strini T, Boon L, Lombardo E, DelaRosa O, Laman JD, Grinyó JM, Weimar W, Betjes MG, Baan CC, Hoogduijn MJ. 2015; Human adipose tissue-derived mesenchymal stem cells abrogate plasmablast formation and induce regulatory B cells indepen-dently of T helper cells. Stem Cells. 33:880–891. DOI: 10.1002/stem.1881. PMID: 25376628. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84923217704&origin=inward.28. Kim DS, Jang IK, Lee MW, Ko YJ, Lee DH, Lee JW, Sung KW, Koo HH, Yoo KH. 2018; Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-γ. EBioMedicine. 28:261–273. DOI: 10.1016/j.ebiom.2018.01.002. PMID: 29366627. PMCID: PMC5898027. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85040649839&origin=inward.29. Putra A, Ridwan FB, Putridewi AI, Kustiyah AR, Wirastuti K, Sadyah NAC, Rosdiana I, Munir D. 2018; The role of TNF-α induced MSCs on suppressive inflammation by increasing TGF-β and IL-10. Open Access Maced J Med Sci. 6:1779–1783. DOI: 10.3889/oamjms.2018.404. PMID: 30455748. PMCID: PMC6236029. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85058173547&origin=inward.30. Wu TY, Liang YH, Wu JC, Wang HS. 2019; Interleukin-1β enhances umbilical cord mesenchymal stem cell adhesion ability on human umbilical vein endothelial cells via LFA-1/ICAM-1 interaction. Stem Cells Int. 2019:7267142. DOI: 10.1155/2019/7267142. PMID: 31949440. PMCID: PMC6948307. PMID: 8df63d2d28d34ace8d0deb3bd318de94. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85077839153&origin=inward.31. Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. 2004; Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 103:4619–4621. DOI: 10.1182/blood-2003-11-3909. PMID: 15001472. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=2942595706&origin=inward.32. Aggarwal S, Pittenger MF. 2005; Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 105:1815–1822. DOI: 10.1182/blood-2004-04-1559. PMID: 15494428. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=13544249606&origin=inward.33. Bauer TM, Jiga LP, Chuang JJ, Randazzo M, Opelz G, Terness P. 2005; Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transpl Int. 18:95–100. DOI: 10.1111/j.1432-2277.2004.00031.x. PMID: 15612990. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=21244487758&origin=inward.34. English K, Barry FP, Field-Corbett CP, Mahon BP. 2007; IFN-gamma and TNF-alpha differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol Lett. 110:91–100. DOI: 10.1016/j.imlet.2007.04.001. PMID: 17507101. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34249662219&origin=inward.35. Massagué J, Like B. 1985; Cellular receptors for type beta transforming growth factor. Ligand binding and affinity labeling in human and rodent cell lines. J Biol Chem. 260:2636–2645. DOI: 10.1016/S0021-9258(18)89408-X. PMID: 2982829.36. Tan JC, Indelicato SR, Narula SK, Zavodny PJ, Chou CC. 1993; Characterization of interleukin-10 receptors on human and mouse cells. J Biol Chem. 268:21053–21059. DOI: 10.1016/S0021-9258(19)36892-9. PMID: 8407942.37. Luk F, Carreras-Planella L, Korevaar SS, de Witte SFH, Borràs FE, Betjes MGH, Baan CC, Hoogduijn MJ, Franquesa M. 2017; Inflammatory conditions dictate the effect of mesenchymal stem or stromal cells on B cell function. Front Immunol. 8:1042. DOI: 10.3389/fimmu.2017.01042. PMID: 28894451. PMCID: PMC5581385. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85028442641&origin=inward.38. Leibacher J, Henschler R. 2016; Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 7:7. DOI: 10.1186/s13287-015-0271-2. PMID: 26753925. PMCID: PMC4709937. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84954101786&origin=inward.39. Braid LR, Wood CA, Wiese DM, Ford BN. 2018; Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes. Cytotherapy. 20:232–244. DOI: 10.1016/j.jcyt.2017.09.013. PMID: 29167063. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85034578828&origin=inward.40. Toupet K, Maumus M, Peyrafitte JA, Bourin P, van Lent PL, Ferreira R, Orsetti B, Pirot N, Casteilla L, Jorgensen C, Noël D. 2013; Long-term detection of human adipose-derived mesenchymal stem cells after intraarticular injection in SCID mice. Arthritis Rheum. 65:1786–1794. DOI: 10.1002/art.37960. PMID: 23553439.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent Trends and Strategies in Stem Cell Therapy for Alzheimer's Disease
- Effect of Capsaicin on Immune Responses, Anaphylaxis and Tumorigenesis in Mice
- mesenchymal stem cells and osteogenesis
- Effects of Human Seminal Plasma on Humoral and Cellular Immune Response in Mice
- Therapeutic Aspects of Mesenchymal Stem Cell-Based Cell Therapy with a Focus on Human Amniotic Epithelial Cells in Multiple Sclerosis: A Mechanistic Review