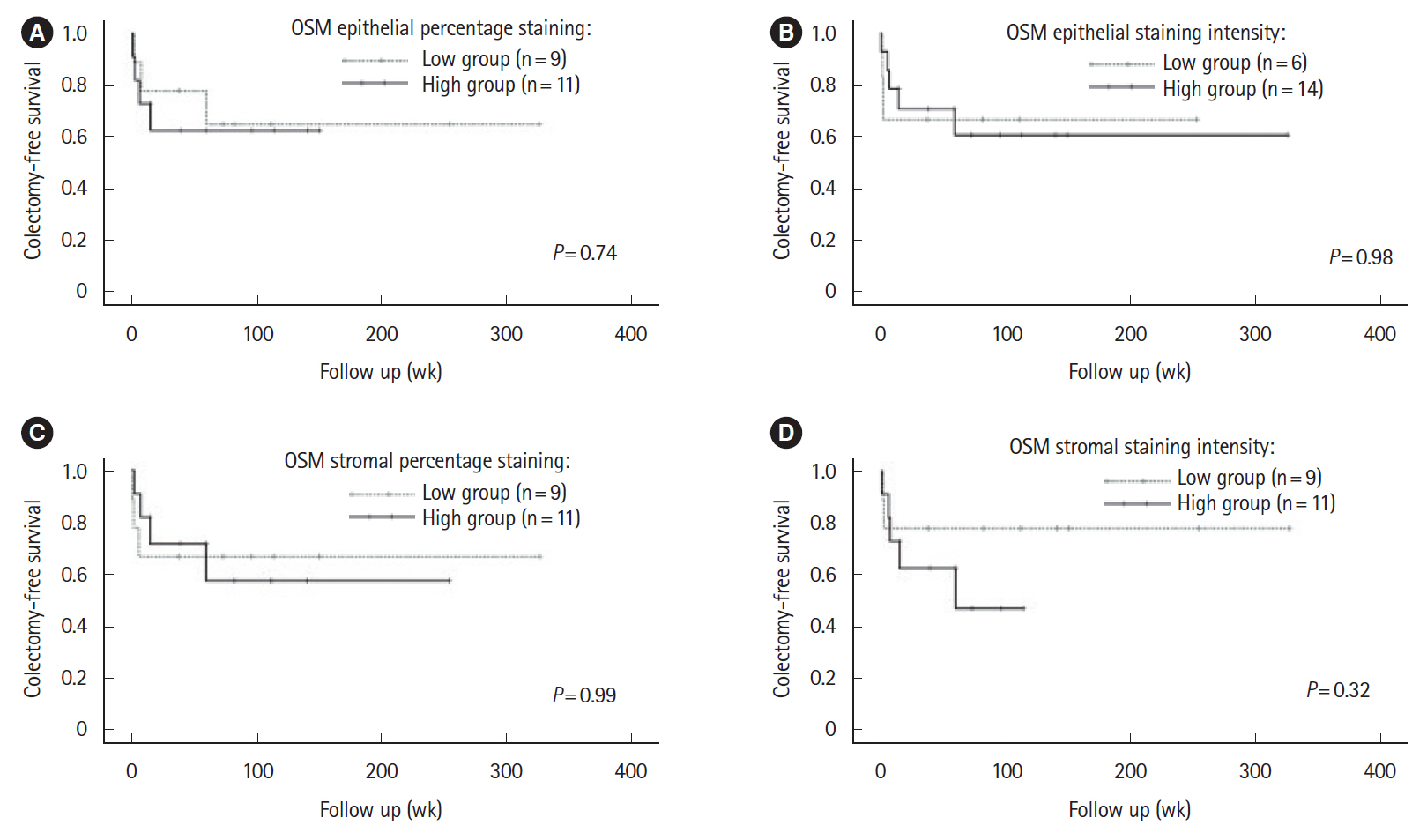
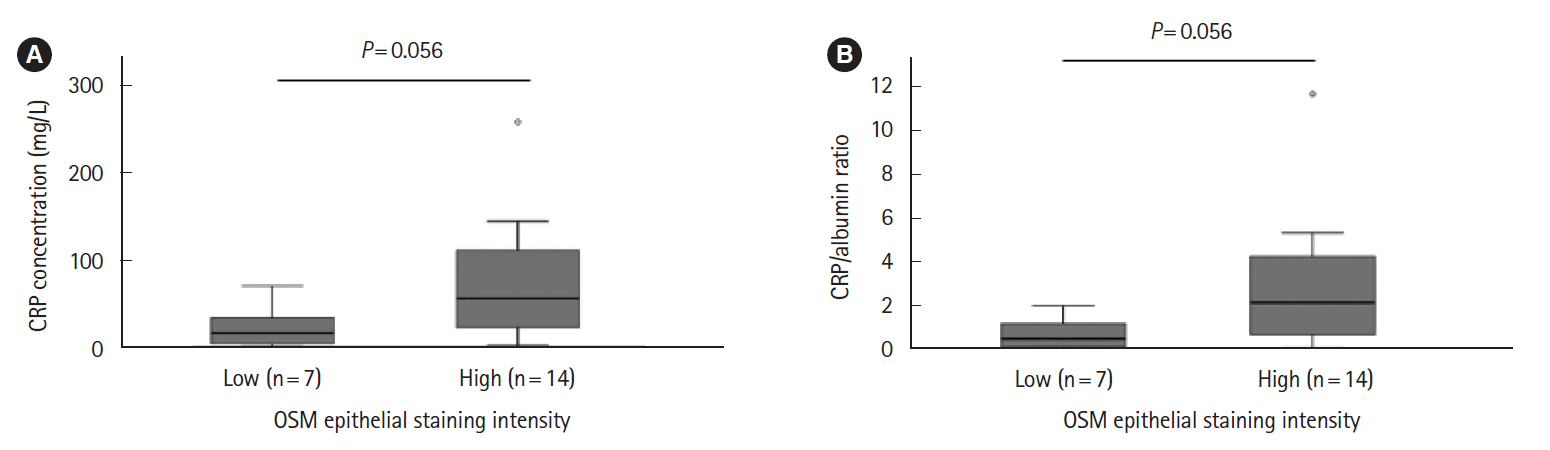
Intest Res.
2022 Jul;20(3):381-385. 10.5217/ir.2021.00073.
Colonic oncostatin M expression evaluated by immunohistochemistry and infliximab therapy outcome in corticosteroid-refractory acute severe ulcerative colitis
- Affiliations
-
- 1Department of Gastroenterology, St James’s Hospital, Dublin, Ireland
- 2Trinity Translational Medicine Institute, Trinity College Dublin, Dublin, Ireland
- 3Department of Histopathology, St James’s Hospital, Dublin, Ireland
- 4Department of Surgery, St James’s Hospital, Dublin, Ireland
- KMID: 2531992
- DOI: http://doi.org/10.5217/ir.2021.00073
Figure
Reference
-
1. Daperno M, Sostegni R, Rocca R, et al. Review article: medical treatment of severe ulcerative colitis. Aliment Pharmacol Ther. 2002; 16 Suppl 4:7–12.2. Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017; 11:769–784.
Article3. Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007; 5:103–110.
Article4. Williams JG, Alam MF, Alrubaiy L, et al. Infliximab versus ciclosporin for steroid-resistant acute severe ulcerative colitis (CONSTRUCT): a mixed methods, open-label, pragmatic randomised trial. Lancet Gastroenterol Hepatol. 2016; 1:15–24.
Article5. Beigel F, Friedrich M, Probst C, et al. Oncostatin M mediates STAT3-dependent intestinal epithelial restitution via increased cell proliferation, decreased apoptosis and upregulation of SERPIN family members. PLoS One. 2014; 9:e93498.
Article6. West NR, Hegazy AN, Owens BM, et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat Med. 2017; 23:579–589.7. Verstockt S, Verstockt B, Machiels K, et al. Oncostatin M is a biomarker of diagnosis, worse disease prognosis, and therapeutic nonresponse in inflammatory bowel disease. Inflamm Bowel Dis. 2021; 27:1564–1575.
Article8. Gibson DJ, Hartery K, Doherty J, et al. CRP/albumin ratio: an early predictor of steroid responsiveness in acute severe ulcerative colitis. J Clin Gastroenterol. 2018; 52:e48–e52.9. Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353:2462–2476.
Article10. Sandborn WJ, Feagan BG, Marano C, et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014; 146:85–95.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advances in ulcerative colitis therapy
- Infliximab versus Cyclosporine Treatment for Severe Corticosteroid-Refractory Ulcerative Colitis: A Korean, Retrospective, Single Center Study
- What to do when traditional rescue therapies fail in acute severe ulcerative colitis
- Does the Cyclosporine Still Have a Potential Role in the Treatment of Acute Severe Steroid-Refractory Ulcerative Colitis?
- Efficacy of Infliximab Rescue Therapy in Hospitalized Patients with Steroid-Refractory Ulcerative Colitis: Single Center Experience