Intest Res.
2022 Jul;20(3):321-328. 10.5217/ir.2021.00022.
Evaluation of nutritional status using bioelectrical impedance analysis in patients with inflammatory bowel disease
- Affiliations
-
- 1Department of Internal Medicine, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea
- KMID: 2531986
- DOI: http://doi.org/10.5217/ir.2021.00022
Abstract
- Background/Aims
Nutritional status influences quality of life among patients with inflammatory bowel disease (IBD), although there is no clear method to evaluate nutritional status in this setting. Therefore, this study examined whether bioelectrical impedance analysis (BIA) could be used to evaluate the nutritional status of patients with IBD.
Methods
We retrospectively analyzed data from 139 Korean patients with IBD who were treated between November 2018 and November 2019. Patients were categorized as having active or inactive IBD based on the Harvey-Bradshaw index (a score of ≥5 indicates active Crohn’s disease) and the partial Mayo scoring index (a score of ≥2 indicates active ulcerative colitis). BIA results and serum nutritional markers were analyzed according to disease activity.
Results
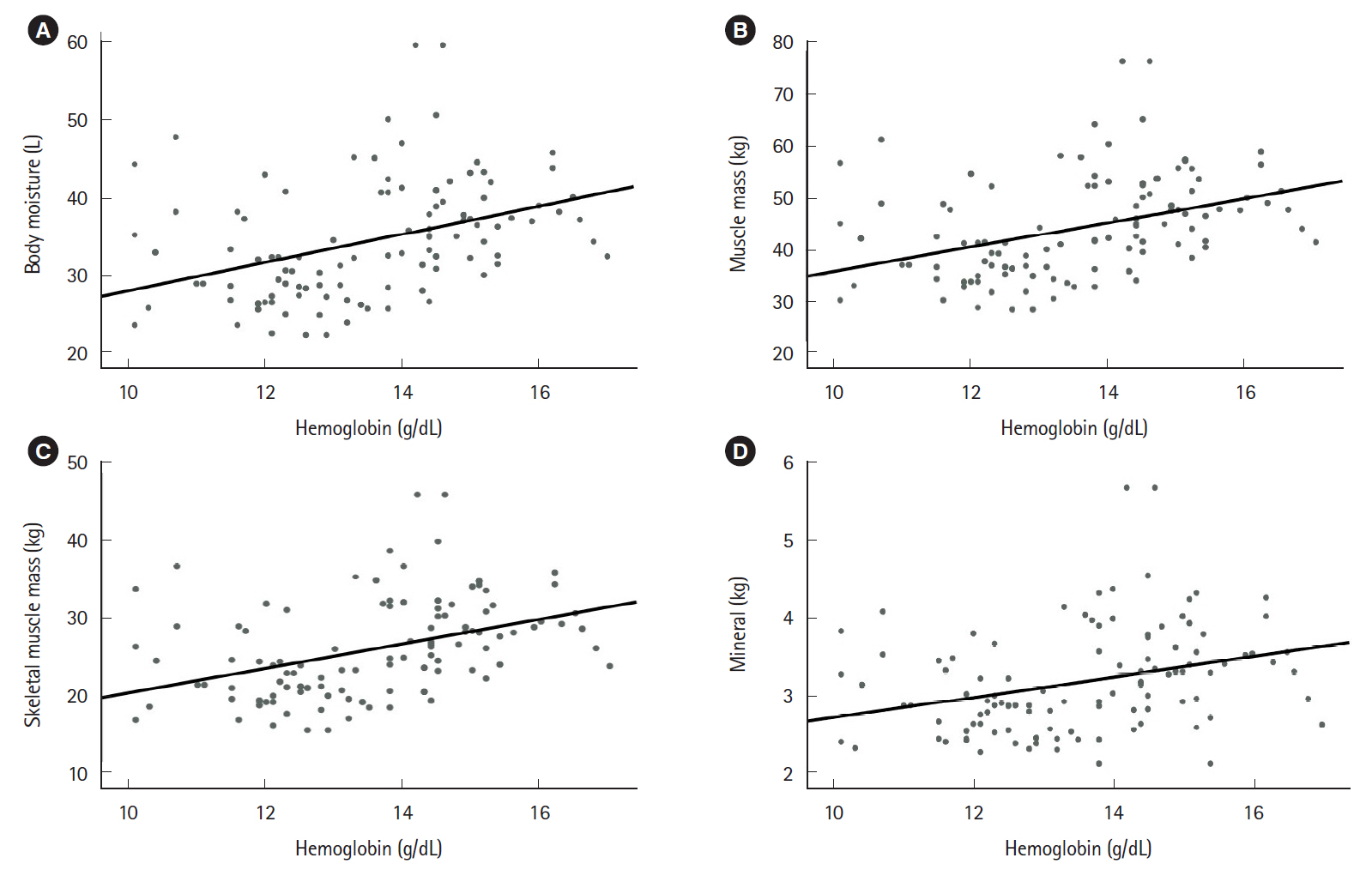
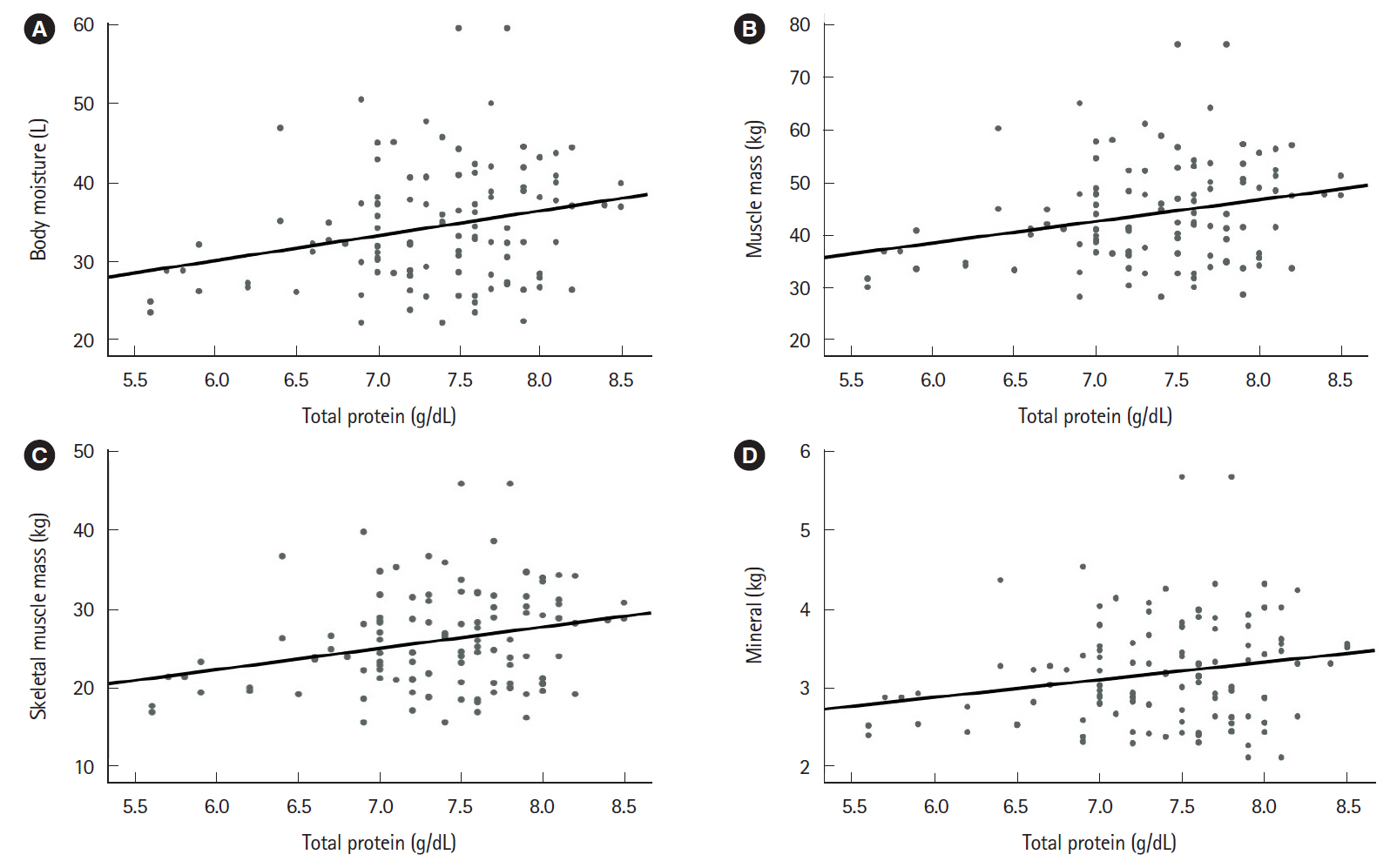
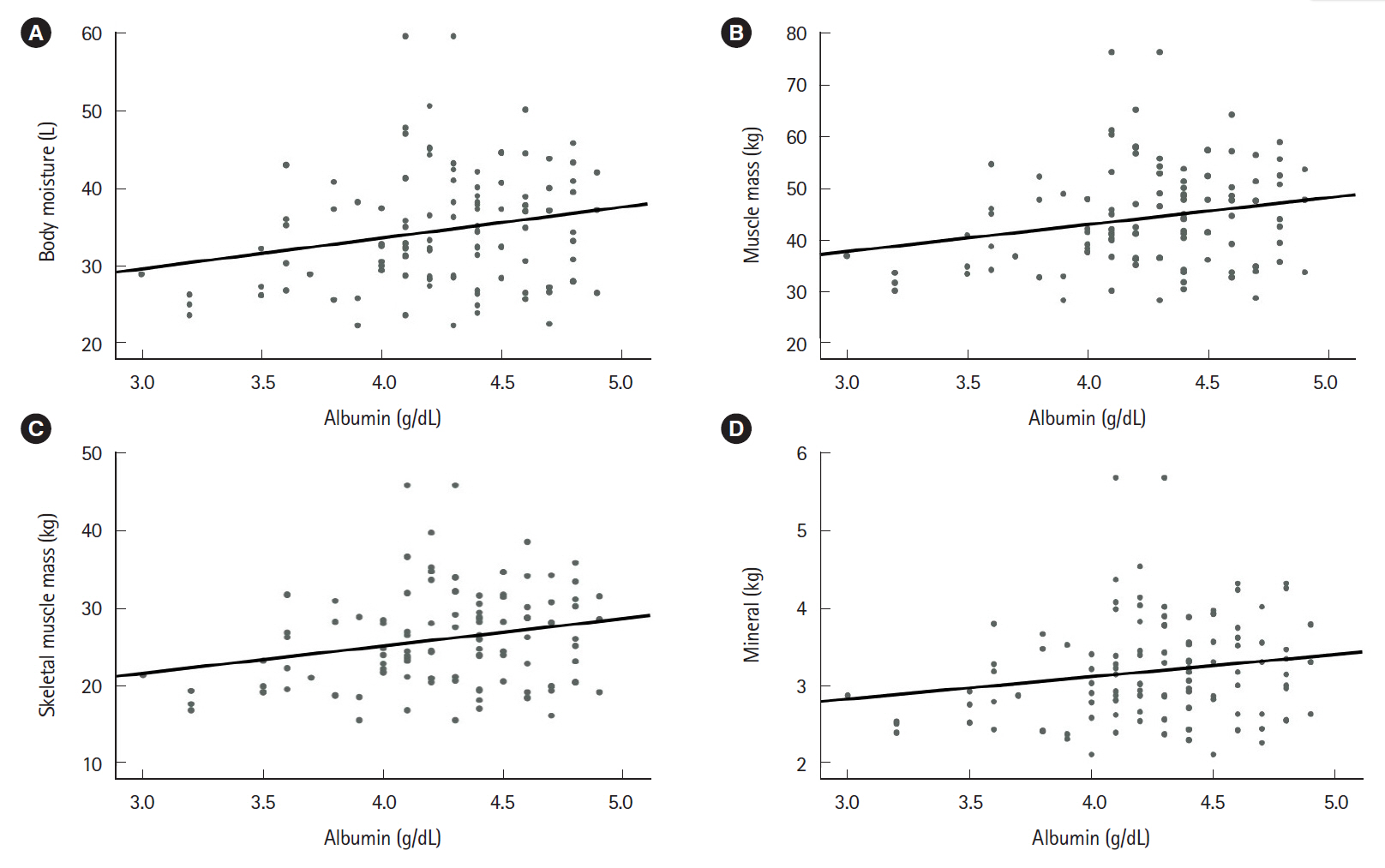
The mean patient age was 45.11±17.71 years. The study included 47 patients with ulcerative colitis and 92 patients with Crohn’s disease. Relative to the group with active disease (n=72), the group with inactive disease (n=67) had significantly higher values for hemoglobin (P<0.001), total protein (P<0.001), and albumin (P<0.001). Furthermore, the group with inactive disease had higher BIA values for body moisture (P=0.047), muscle mass (P=0.046), skeletal muscle mass (P=0.042), body mass index (P=0.027), and mineral content (P=0.034). Moreover, the serum nutritional markers were positively correlated with the BIA results.
Conclusions
Nutritional markers evaluated using BIA were correlated with serum nutritional markers and inversely correlated with disease activity. Therefore, we suggest that BIA may be a useful tool that can help existing nutritional tests monitor the nutritional status of IBD patients.
Keyword
Figure
Reference
-
1. Alatab S, Sepanlou SG, Ikuta K, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020; 5:17–30.2. Kim ES, Kim WH. Inflammatory bowel disease in Korea: epidemiological, genomic, clinical, and therapeutic characteristics. Gut Liver. 2010; 4:1–14.
Article3. Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res. 2016; 14:111–119.
Article4. Kaibullayeva J, Ualiyeva A, Oshibayeva A, Dushpanova A, Marshall JK. Prevalence and patient awareness of inflammatory bowel disease in Kazakhstan: a cross-sectional study. Intest Res. 2020; 18:430–437.
Article5. Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014; 20:91–99.
Article6. Owczarek D, Rodacki T, Domagała-Rodacka R, Cibor D, Mach T. Diet and nutritional factors in inflammatory bowel diseases. World J Gastroenterol. 2016; 22:895–905.
Article7. Cohen AB, Lee D, Long MD, et al. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig Dis Sci. 2013; 58:1322–1328.
Article8. Yoon JY. Nutritional approach as therapeutic manipulation in inflammatory bowel disease. Intest Res. 2019; 17:463–475.
Article9. Shafiee NH, Manaf ZA, Mokhtar NM, Raja Ali RA. An assessment of dietary intake, food avoidance and food beliefs in patients with ulcerative colitis of different disease status. Intest Res. 2020; 18:447–458.
Article10. Ko KH, Kim YS, Lee BK, et al. Vitamin D deficiency is associated with disease activity in patients with Crohn’s disease. Intest Res. 2019; 17:70–77.
Article11. Green N, Miller T, Suskind D, Lee D. A review of dietary therapy for IBD and a vision for the future. Nutrients. 2019; 11:947.
Article12. Mentella MC, Scaldaferri F, Pizzoferrato M, Gasbarrini A, Miggiano GA. Nutrition, IBD and gut microbiota: a review. Nutrients. 2020; 12:944.
Article13. Kim SE. Nutritional screening and assessment in hospitalized patients. Korean J Gastroenterol. 2015; 65:336–341.
Article14. Antonio J, Kenyon M, Ellerbroek A, et al. Body composition assessment: a comparison of the bod pod, InBody 770, and DXA. J Exerc Nutr. 2019; 2:11.15. Marra M, Sammarco R, De Lorenzo A, et al. Assessment of body composition in health and disease using bioelectrical impedance analysis (BIA) and dual energy X-ray absorptiometry (DXA): a critical overview. Contrast Media Mol Imaging. 2019; 2019:3548284.
Article16. Bhattacharya A, Pal B, Mukherjee S, Roy SK. Assessment of nutritional status using anthropometric variables by multivariate analysis. BMC Public Health. 2019; 19:1045.
Article17. Krel C, Piko N, Tomazic J, Bevc S. Bioelectrical impedance analysis as a marker of nutritional status in chronically ill patients. Aust J Adv Nurs. 2019; 36:14–21.18. Bryant RV, Trott MJ, Bartholomeusz FD, Andrews JM. Systematic review: body composition in adults with inflammatory bowel disease. Aliment Pharmacol Ther. 2013; 38:213–225.
Article19. Zalizko P, Roshofa TH, Meija L, Bodnieks E, Pukitis A. The role of body muscle mass as an indicator of activity in inflammatory bowel disease patients. Clin Nutr ESPEN. 2020; 40:193–200.
Article20. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis: a randomized study. N Engl J Med. 1987; 317:1625–1629.
Article21. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980; 1:514.
Article22. Alhagamhmad MH, Day AS, Lemberg DA, Leach ST. An update of the role of nutritional therapy in the management of Crohn’s disease. J Gastroenterol. 2012; 47:872–882.
Article23. Cioffi I, Imperatore N, Di Vincenzo O, et al. Association between health-related quality of life and nutritional status in adult patients with Crohn’s disease. Nutrients. 2020; 12:746.
Article24. Mijac DD, Janković GL, Jorga J, Krstić MN. Nutritional status in patients with active inflammatory bowel disease: prevalence of malnutrition and methods for routine nutritional assessment. Eur J Intern Med. 2010; 21:315–319.
Article25. Kuchnia A, Earthman C, Teigen L, et al. Evaluation of bioelectrical impedance analysis in critically ill patients: results of a multicenter prospective study. JPEN J Parenter Enteral Nutr. 2017; 41:1131–1138.
Article26. Wilson FP, Xie D, Anderson AH, et al. Urinary creatinine excretion, bioelectrical impedance analysis, and clinical outcomes in patients with CKD: the CRIC study. Clin J Am Soc Nephrol. 2014; 9:2095–2103.
Article27. Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015; 6:62–72.
Article28. Powell-Tuck J. Protein metabolism in inflammatory bowel disease. Gut. 1986; 27 Suppl 1(Suppl 1):67–71.
Article29. Kelsen J, Baldassano RN. Inflammatory bowel disease: the difference between children and adults. Inflamm Bowel Dis. 2008; 14 Suppl 2:S9–S11.
Article30. Farthing M, Salam MA, Lindberg G, et al. Acute diarrhea in adults and children: a global perspective. J Clin Gastroenterol. 2013; 47:12–20.31. Alkhouri RH, Hashmi H, Baker RD, Gelfond D, Baker SS. Vitamin and mineral status in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013; 56:89–92.
Article32. Ohlund I, Hernell O, Hörnell A, Stenlund H, Lind T. BMI at 4 years of age is associated with previous and current protein intake and with paternal BMI. Eur J Clin Nutr. 2010; 64:138–145.
Article33. Bijelic R, Balaban J, Milicevic S. Correlation of the lipid profile, BMI and bone mineral density in postmenopausal women. Mater Sociomed. 2016; 28:412–415.
Article34. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019; 13:144–164.
Article35. Shiga H, Abe I, Onodera M, et al. Serum C-reactive protein and albumin are useful biomarkers for tight control management of Crohn’s disease in Japan. Sci Rep. 2020; 10:511.
Article36. Agouridis AP, Elisaf M, Milionis HJ. An overview of lipid abnormalities in patients with inflammatory bowel disease. Ann Gastroenterol. 2011; 24:181–187.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nutritional Support in Patients with Inflammatory Bowel Diseases
- Percent of Body Fat by Bioelectrical Impedance Analysis in Healthy Children
- The Pharmacotherapy of Inflammatory Bowel Disease in Child and Adolescence
- Nutritional aspect of pediatric inflammatory bowel disease: its clinical importance
- Nutritional concerns in pediatric inflammatory bowel disease