Endocrinol Metab.
2022 Jun;37(3):497-505. 10.3803/EnM.2022.1427.
Real-World Safety and Effectiveness of Denosumab in Patients with Osteoporosis: A Prospective, Observational Study in South Korea
- Affiliations
-
- 1Department of Internal Medicine, Severance Hospital, Endocrine Research Institute, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Orthopedic Surgery, Inje University Sanggye Paik Hospital, College of Medicine, Inje University, Seoul, Korea
- 3Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Amgen Korea Ltd., Seoul, Korea
- 5Division of Endocrinology and Metabolism, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2531393
- DOI: http://doi.org/10.3803/EnM.2022.1427
Abstract
- Background
The efficacy and safety of denosumab have been established in a phase 3, randomized, placebo-controlled trial in Korean postmenopausal women with osteoporosis. This postmarketing surveillance study was aimed to investigate the safety and effectiveness of denosumab in Korean real-world clinical practice.
Methods
Patients with osteoporosis who had received denosumab per the Korean approved indications in the postmarketing setting between September 2014 and September 2019 were enrolled. The primary endpoint was the incidence of adverse events (AEs) and adverse drug reactions (ADRs). The secondary endpoint was the percent change from baseline in bone mineral density (BMD) of the lumbar spine, total hip, and femoral neck.
Results
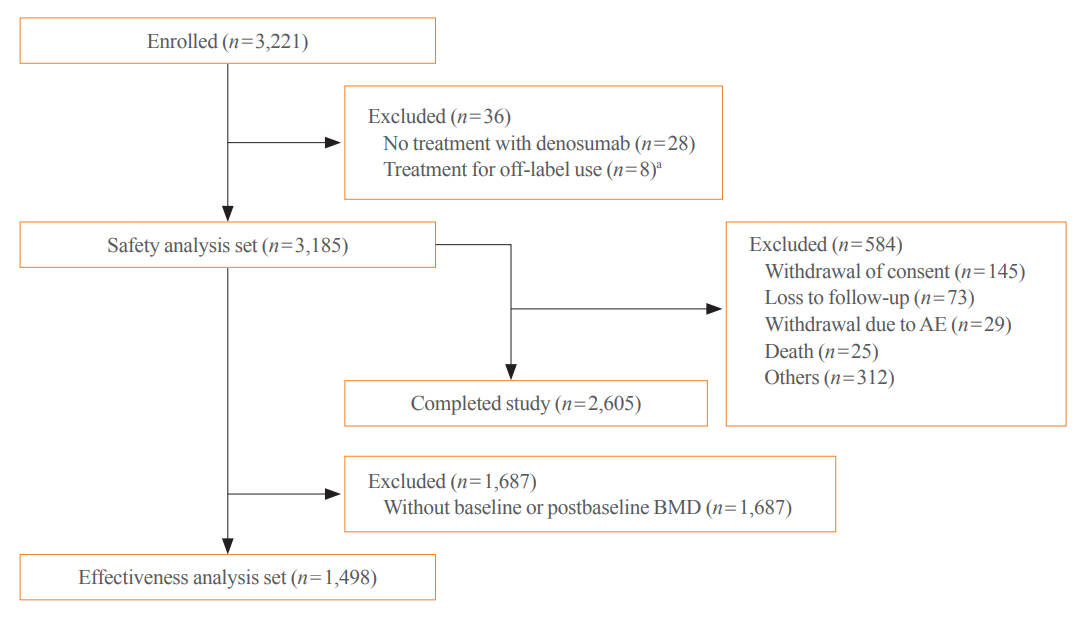
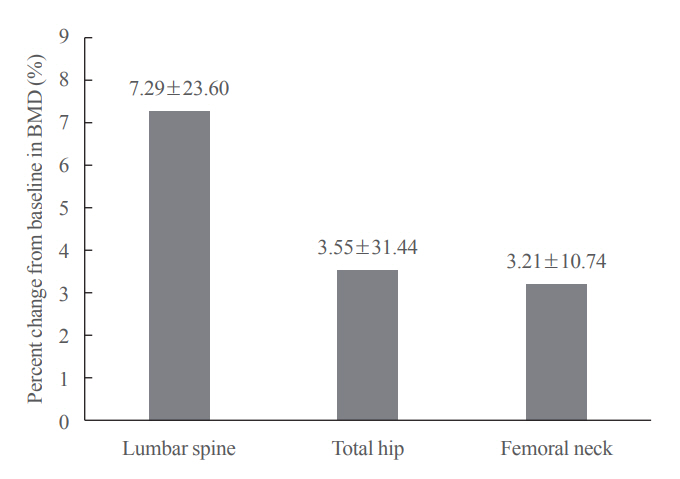
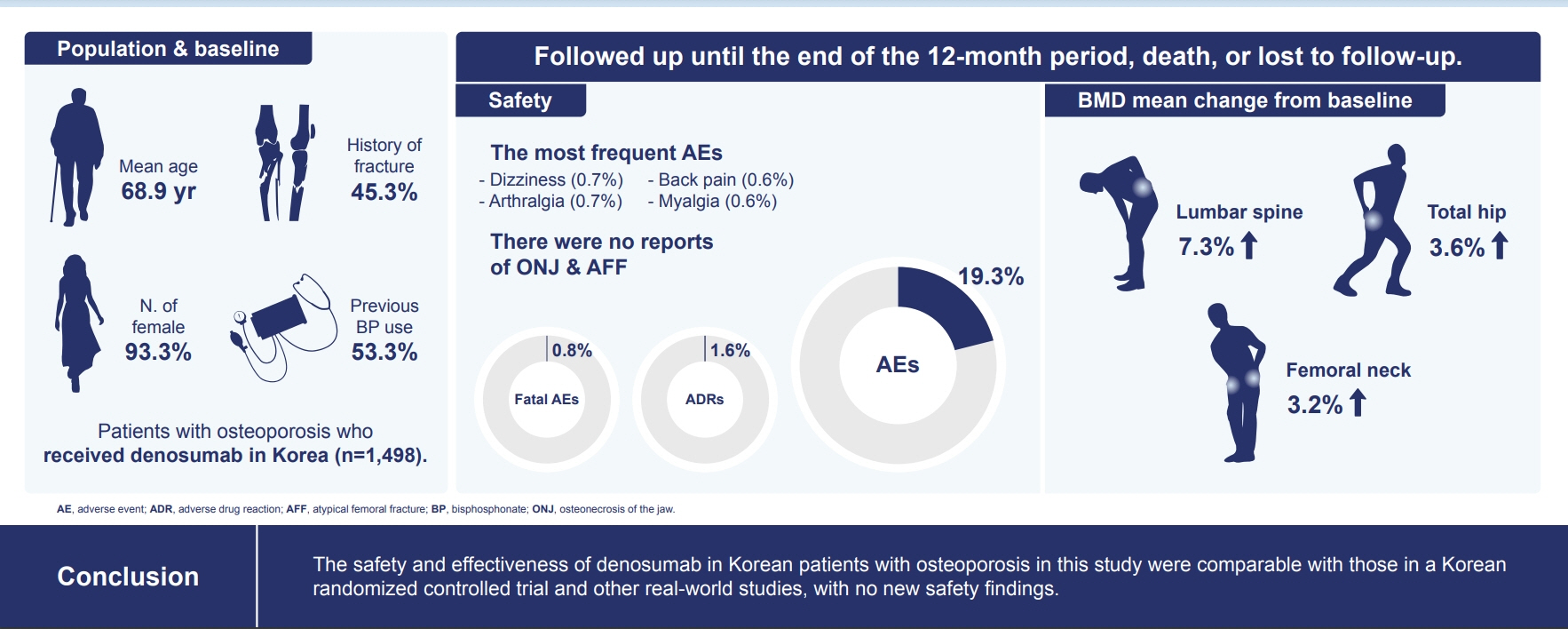
Of the 3,221 patients enrolled, 3,185 were included in the safety analysis set; 2,973 (93.3%) were female, and the mean± standard deviation (SD) age was 68.9±9.9 years. The mean±SD study period was 350.0±71.4 days. AEs, fatal AEs, and ADRs occurred in 19.3%, 0.8%, and 1.6%, respectively. The most frequent AEs, occurring in >0.5% of patients, were dizziness (0.7%), arthralgia (0.7%), back pain (0.6%), and myalgia (0.6%). Hypocalcemia occurred in 0.3% of patients. There were no cases of osteonecrosis of the jaw and atypical femoral fracture. Mean±SD percent change from baseline in BMD of the lumbar spine, total hip, and femoral neck was 7.3%±23.6%, 3.6%±31.4%, and 3.2%±10.7%, respectively.
Conclusion
The safety and effectiveness of denosumab in Korean patients with osteoporosis in this study were comparable with those in the Korean randomized controlled trial, with no new safety findings.
Figure
Cited by 2 articles
-
Persistence with Denosumab in Male Osteoporosis Patients: A Real-World, Non-Interventional Multicenter Study
Chaiho Jeong, Jeongmin Lee, Jinyoung Kim, Jeonghoon Ha, Kwanhoon Jo, Yejee Lim, Mee Kyoung Kim, Hyuk-Sang Kwon, Tae-Seo Sohn, Ki-Ho Song, Moo Il Kang, Ki-Hyun Baek
Endocrinol Metab. 2023;38(2):260-268. doi: 10.3803/EnM.2023.1663.Long-Term Efficacy and Safety of Denosumab: Insights beyond 10 Years of Use
Jeonghoon Ha, Youn-Ju Lee, Jinyoung Kim, Chaiho Jeong, Yejee Lim, Jeongmin Lee, Ki-Hyun Baek
Endocrinol Metab. 2025;40(1):47-56. doi: 10.3803/EnM.2024.2125.
Reference
-
1. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993; 94:646–50.2. Harris JR, Korolchuk VI. Biochemistry and cell biology of ageing: Part II clinical science. Subcellular Biochemistry. Singapore: Springer;2019. Chapter 16, Osteoporosis and the ageing skeleton. p. 453–76.3. Ballane G, Cauley JA, Luckey MM, El-Hajj Fuleihan G. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos Int. 2017; 28:1531–42.
Article4. Yoon DS, Lee YK, Ha YC, Kim HY. Inadequate dietary calcium and vitamin D intake in patients with osteoporotic fracture. J Bone Metab. 2016; 23:55–61.
Article5. Ahn SH, Park SM, Park SY, Yoo JI, Jung HS, Nho JH, et al. Osteoporosis and osteoporotic fracture fact sheet in Korea. J Bone Metab. 2020; 27:281–90.
Article6. Park EJ, Joo IW, Jang MJ, Kim YT, Oh K, Oh HJ. Prevalence of osteoporosis in the Korean population based on Korea National Health and Nutrition Examination Survey (KNHANES), 2008-2011. Yonsei Med J. 2014; 55:1049–57.
Article7. Deeks ED. Denosumab: a review in postmenopausal osteoporosis. Drugs Aging. 2018; 35:163–73.
Article8. Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009; 361:756–65.
Article9. Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, et al. 10 Years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017; 5:513–23.
Article10. Koh JM, Chung DJ, Chung YS, Kang MI, Kim IJ, Min YK, et al. Assessment of denosumab in Korean postmenopausal women with osteoporosis: randomized, double-blind, placebo-controlled trial with open-label extension. Yonsei Med J. 2016; 57:905–14.
Article11. AMGEN. Prolia® Pre-filled Syringe [Internet]. Seoul: AMGEN;2022. [cited 2022 May 10]. Available from: https://www.amgen.co.kr/products/prolia.12. Health Insurance Review & Assessment Service. [Pharmaceuticals] Notification No. 2019-57 Detailed information on the application standards and methods of medical care benefits [Internet]. Wonju: HIRA;2019. [cited 2022 May 10]. Available from: https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020002000100&brdScnBltNo=4&brdBltNo=7268&pageIndex=145.13. Shoback D, Rosen CJ, Black DM, Cheung AM, Murad MH, Eastell R. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society guideline update. J Clin Endocrinol Metab. 2020; 105:dgaa048.
Article14. Tanaka S, Mizutani H, Tsuruya E, Fukuda R, Kuge K, Okubo N. Long-term safety and effectiveness of denosumab in Japanese patients with osteoporosis: 3-year post-marketing surveillance study. J Bone Miner Metab. 2021; 39:463–73.
Article15. MedDRA. Introductory guide MedDRA version 22.1 [Internet]. Herndon: MedDRA;2019. [cited 2022 May 10]. Available from: https://admin.meddra.org/sites/default/files/guidance/file/000354_intguide_22.1.pdf.16. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019; 393:364–76.
Article17. Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017; 5:898–907.
Article18. Lv F, Cai X, Yang W, Gao L, Chen L, Wu J, et al. Denosumab or romosozumab therapy and risk of cardiovascular events in patients with primary osteoporosis: systematic review and meta-analysis. Bone. 2020; 130:115121.19. Kim KJ, Hong N, Lee S, Kim M, Rhee Y. A simple-to-use score for identifying individuals at high risk of denosumab-associated hypocalcemia in postmenopausal osteoporosis: a real-world cohort study. Calcif Tissue Int. 2020; 107:567–75.
Article20. Tsourdi E, Zillikens MC, Meier C, Body JJ, Gonzalez Rodriguez E, Anastasilakis AD, et al. Fracture risk and management of discontinuation of denosumab therapy: a systematic review and position statement by ECTS. J Clin Endocrinol Metab. 2021; 106:264–81.
Article21. Symonds C, Kline G. Warning of an increased risk of vertebral fracture after stopping denosumab. CMAJ. 2018; 190:E485–6.
Article22. Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, et al. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2018; 33:190–8.
Article23. Kim BK, Kim CH, Min YK. Preventing rebound-associated fractures after discontinuation of denosumab therapy: a position statement from the health insurance committee of the Korean Endocrine Society. Endocrinol Metab (Seoul). 2021; 36:909–11.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Denosumab for the treatment of osteoporosis
- Denosumab (RANKL Inhibitor): A Potent Anti-Resorptive Agent
- Long-Term Efficacy and Safety of Denosumab: Insights beyond 10 Years of Use
- Cost-Effectiveness of Denosumab for Post-Menopausal Osteoporosis in South Korea
- Cost-Effectiveness of Denosumab for the Treatment of Postmenopausal Osteoporosis in South Korea