Endocrinol Metab.
2022 Jun;37(3):455-465. 10.3803/EnM.2022.1434.
The Impact of Insulin Resistance on Hepatic Fibrosis among United States Adults with Non-Alcoholic Fatty Liver Disease: NHANES 2017 to 2018
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea
- 2General Medicine Service, Veterans Affairs Puget Sound Health Care System, University of Washington, Seattle, WA, USA
- 3Division of Endocrinology, Veterans Affairs Puget Sound Health Care System, Seattle, WA, USA
- 4Division of Metabolism, Endocrinology and Nutrition, Department of Medicine, University of Washington, Seattle, WA, USA
- KMID: 2531389
- DOI: http://doi.org/10.3803/EnM.2022.1434
Abstract
- Background
We aimed to investigate the association of hepatic steatosis with liver fibrosis and to assess the interactive effects of hepatic steatosis and insulin resistance on liver fibrosis in a nationally representative sample of United States adults.
Methods
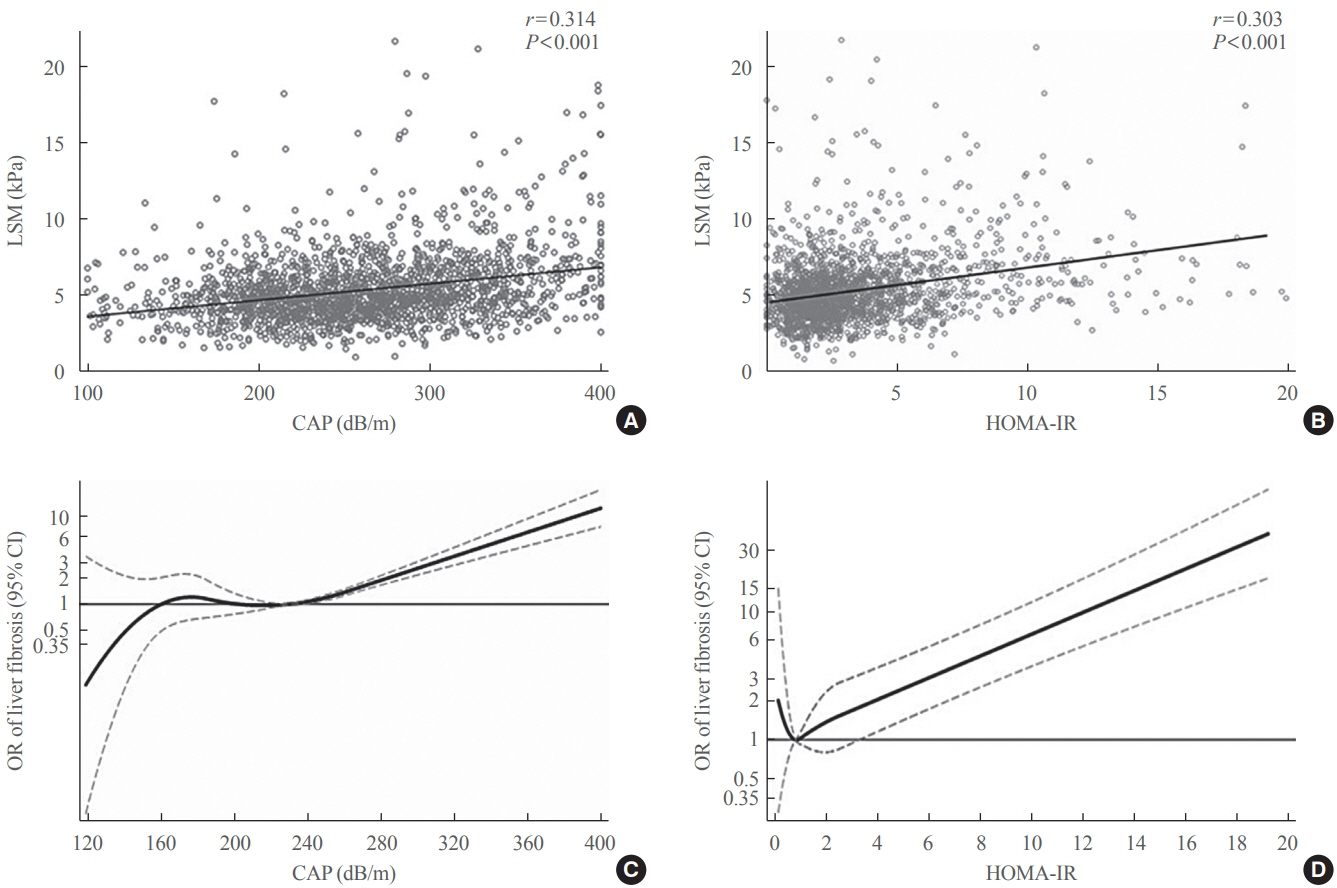
We conducted a cross-sectional analysis using data from National Health and Nutrition Examination Survey 2017 to 2018, which for the first time included transient elastography to assess liver stiffness and hepatic steatosis. We evaluated the association between hepatic steatosis (using controlled attenuation parameter [CAP]) and clinically significant liver fibrosis (defined as liver stiffness ≥7.5 kPa) using logistic regression with an interaction term for hepatic steatosis and insulin resistance (defined as homeostatic model assessment of insulin resistance ≥3.0).
Results
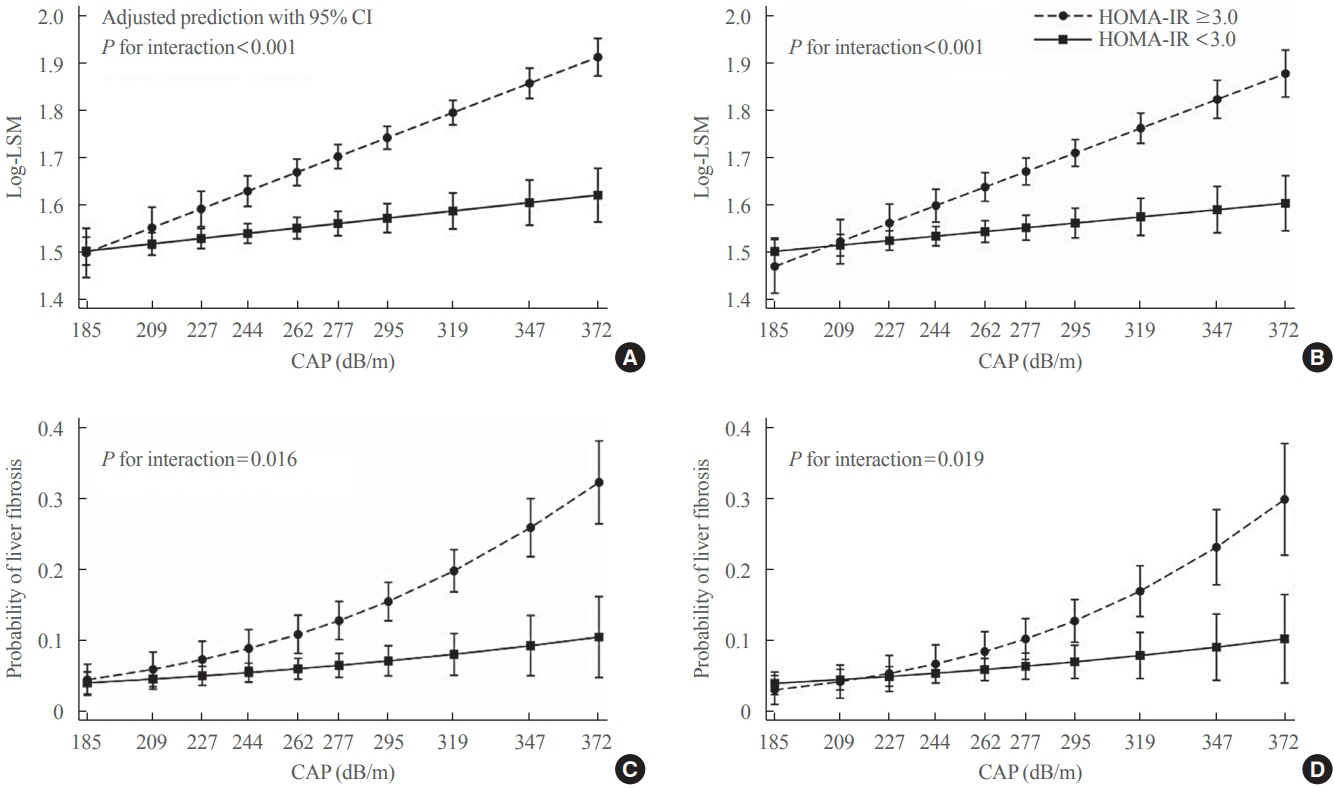
Among adults undergoing transient elastography (n=2,023), 45.9% had moderate or greater hepatic steatosis and 11.3% had clinically significant liver fibrosis. After adjustment for demographic and metabolic factors, the odds of significant liver fibrosis increased as CAP score rose (odds ratio, 1.35 per standard deviation increment; 95% confidence interval, 1.11 to 1.64). We detected a significant interaction effect between CAP score and insulin resistance on the probability of significant liver fibrosis (P=0.016 for interaction). The probability of significant liver fibrosis increased in the presence of insulin resistance with increasing CAP score, while those without insulin resistance had low probability of significant liver fibrosis, even with high CAP scores.
Conclusion
Individuals with hepatic steatosis had higher odds of fibrosis when insulin resistance was present. Our findings emphasize the importance of the metabolic aspects of the disease on fibrosis risk and suggest a need to better identify patients with metabolic associated fatty liver disease.
Keyword
Figure
Cited by 2 articles
-
DPP-4 Inhibitor in Type 2 Diabetes Mellitus Patient with Non-Alcoholic Fatty Liver Disease: Achieving Two Goals at Once?
Ji Cheol Bae
Endocrinol Metab. 2022;37(6):858-860. doi: 10.3803/EnM.2022.605.Insulin Resistance, Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Clinical and Experimental Perspective
Inha Jung, Dae-Jeong Koo, Won-Young Lee
Diabetes Metab J. 2024;48(3):327-339. doi: 10.4093/dmj.2023.0350.
Reference
-
1. Younossi ZM. Non-alcoholic fatty liver disease: a global public health perspective. J Hepatol. 2019; 70:531–44.2. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018; 15:11–20.
Article3. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016; 64:73–84.
Article4. Rhee EJ. Nonalcoholic fatty liver disease and diabetes: an epidemiological perspective. Endocrinol Metab (Seoul). 2019; 34:226–33.
Article5. Finck BN. Targeting metabolism, insulin resistance, and diabetes to treat nonalcoholic steatohepatitis. Diabetes. 2018; 67:2485–93.
Article6. Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells. 2020; 9:875.
Article7. Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020; 158:1999–2014.
Article8. Bae JC, Cho YK, Lee WY, Seo HI, Rhee EJ, Park SE, et al. Impact of nonalcoholic fatty liver disease on insulin resistance in relation to HbA1c levels in nondiabetic subjects. Am J Gastroenterol. 2010; 105:2389–95.
Article9. Khan RS, Bril F, Cusi K, Newsome PN. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology. 2019; 70:711–24.
Article10. Spengler EK, Loomba R. Recommendations for diagnosis, referral for liver biopsy, and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mayo Clin Proc. 2015; 90:1233–46.
Article11. Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol. 2016; 65:1006–16.
Article12. Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Ledinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017; 66:1022–30.
Article13. European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015; 63:237–64.14. Lee DH. Noninvasive evaluation of nonalcoholic fatty liver disease. Endocrinol Metab (Seoul). 2020; 35:243–59.
Article15. Centers for Disease Control and Prevention, National Center for Health Statistics. National Health and Nutrition Examination Survey [Internet]. Atlanta: CDC;2022. [cited 2022 May 18]. Available from: https://www.cdc.gov/nchs/nhanes/index.htm.16. Boursier J, Zarski JP, de Ledinghen V, Rousselet MC, Sturm N, Lebail B, et al. Determination of reliability criteria for liver stiffness evaluation by transient elastography. Hepatology. 2013; 57:1182–91.
Article17. Inzucchi SE, Viscoli CM, Young LH, Furie KL, Gorman M, Lovejoy AM, et al. Pioglitazone prevents diabetes in patients with insulin resistance and cerebrovascular disease. Diabetes Care. 2016; 39:1684–92.
Article18. Bonder A, Afdhal N. Utilization of FibroScan in clinical practice. Curr Gastroenterol Rep. 2014; 16:372.
Article19. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018; 67:328–57.
Article20. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021; 44(Suppl 1):S15–33.21. Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020; 73:202–9.
Article22. Weinberg EM, Trinh HN, Firpi RJ, Bhamidimarri KR, Klein S, Durlam J, et al. Lean Americans with nonalcoholic fatty liver disease have lower rates of cirrhosis and comorbid diseases. Clin Gastroenterol Hepatol. 2021; 19:996–1008.
Article23. Leung JC, Loong TC, Wei JL, Wong GL, Chan AW, Choi PC, et al. Histological severity and clinical outcomes of nonalcoholic fatty liver disease in nonobese patients. Hepatology. 2017; 65:54–64.
Article24. Wei JL, Leung JC, Loong TC, Wong GL, Yeung DK, Chan RS, et al. Prevalence and severity of nonalcoholic fatty liver disease in non-obese patients: a population study using proton-magnetic resonance spectroscopy. Am J Gastroenterol. 2015; 110:1306–14.
Article25. Fracanzani AL, Petta S, Lombardi R, Pisano G, Russello M, Consonni D, et al. Liver and cardiovascular damage in patients with lean nonalcoholic fatty liver disease, and association with visceral obesity. Clin Gastroenterol Hepatol. 2017; 15:1604–11.
Article26. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000; 106:473–81.
Article27. Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003; 37:917–23.
Article28. Tada T, Toyoda H, Sone Y, Yasuda S, Miyake N, Kumada T, et al. Type 2 diabetes mellitus: a risk factor for progression of liver fibrosis in middle-aged patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2019; 34:2011–8.
Article29. Bjorkstrom K, Franzen S, Eliasson B, Miftaraj M, Gudbjornsdottir S, Trolle-Lagerros Y, et al. Risk factors for severe liver disease in patients with type 2 diabetes. Clin Gastroenterol Hepatol. 2019; 17:2769–75.
Article30. Kim Y, Chang Y, Cho YK, Ahn J, Shin H, Ryu S. Obesity and weight gain are associated with progression of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019; 17:543–50.
Article31. Chen Z, Yu R, Xiong Y, Du F, Zhu S. A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 2017; 16:203.
Article32. Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012; 35:66–75.
Article33. Cusi K, Orsak B, Bril F, Lomonaco R, Hecht J, Ortiz-Lopez C, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016; 165:305–15.
Article34. Gawrieh S, Noureddin M, Loo N, Mohseni R, Awasty V, Cusi K, et al. Saroglitazar, a PPAR-α/γ agonist, for treatment of NAFLD: a randomized controlled double-blind phase 2 trial. Hepatology. 2021; 74:1809–24.
Article35. Goyal O, Nohria S, Goyal P, Kaur J, Sharma S, Sood A, et al. Saroglitazar in patients with non-alcoholic fatty liver disease and diabetic dyslipidemia: a prospective, observational, real world study. Sci Rep. 2020; 10:21117.
Article36. Muller MJ, Willmann O, Rieger A, Fenk A, Selberg O, Lautz HU, et al. Mechanism of insulin resistance associated with liver cirrhosis. Gastroenterology. 1992; 102:2033–41.
Article37. Imano E, Kanda T, Nakatani Y, Motomura M, Arai K, Matsuhisa M, et al. Impaired splanchnic and peripheral glucose uptake in liver cirrhosis. J Hepatol. 1999; 31:469–73.
Article38. Chung W, Promrat K, Wands J. Clinical implications, diagnosis, and management of diabetes in patients with chronic liver diseases. World J Hepatol. 2020; 12:533–57.
Article39. Parvaiz F, Manzoor S, Tariq H, Javed F, Fatima K, Qadri I. Hepatitis C virus infection: molecular pathways to insulin resistance. Virol J. 2011; 8:474.
Article40. Chen YM, Zhao JF, Liu YL, Chen J, Jiang RL. Chronic ethanol treatment of human hepatocytes inhibits the activation of the insulin signaling pathway by increasing cytosolic free calcium levels. Int J Mol Med. 2015; 36:739–46.
Article41. Chiang DJ, Pritchard MT, Nagy LE. Obesity, diabetes mellitus, and liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2011; 300:G697–702.
Article42. Malaguarnera M, Di Rosa M, Nicoletti F, Malaguarnera L. Molecular mechanisms involved in NAFLD progression. J Mol Med (Berl). 2009; 87:679–95.
Article43. Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, et al. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001; 34(4 Pt 1):738–44.
Article44. Fujii H, Imajo K, Yoneda M, Nakahara T, Hyogo H, Takahashi H, et al. HOMA-IR: an independent predictor of advanced liver fibrosis in nondiabetic non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2019; 34:1390–5.
Article45. Aller R, Siguenza R, Pina M, Laserna C, Antolin B, Burgueno B, et al. Insulin resistance is related with liver fibrosis in type 2 diabetic patients with non-alcoholic fatty liver disease proven biopsy and Mediterranean diet pattern as a protective factor. Endocrine. 2020; 68:557–63.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The SCAP/SREBP Pathway: A Mediator of Hepatic Steatosis
- The Progression of Liver Fibrosis in Non-alcoholic Fatty Liver Disease
- Clinical impact of nonalcoholic fatty liver disease on other medical diseases
- Definition, Pathogenesis, and Natural Progress of Non-alcoholic Fatty Liver Disease
- Implications of Mitochondrial Unfolded Protein Response and Mitokines: A Perspective on Fatty Liver Diseases