Cancer Res Treat.
2022 Jul;54(3):728-736. 10.4143/crt.2021.480.
Cost Utility Analysis of a Pilot Study for the Korean Lung Cancer Screening Project
- Affiliations
-
- 1Asan Medical Institute of Convergence Science and Technology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Preventive Medicine, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Nursing, College of Nursing, Dankook University, Cheonan, Korea
- 4Department of Pulmonology and Critical Care Medicine, Asan Medical Center, College of Medicine, University of Ulsan, Seoul, Korea
- 5National Cancer Control Institute, National Cancer Center, Goyang, Korea
- KMID: 2531319
- DOI: http://doi.org/10.4143/crt.2021.480
Abstract
- Purpose
The aim of this study was to evaluate the cost utility of a pilot study of Korean Lung Cancer Screening Project.
Materials and Methods
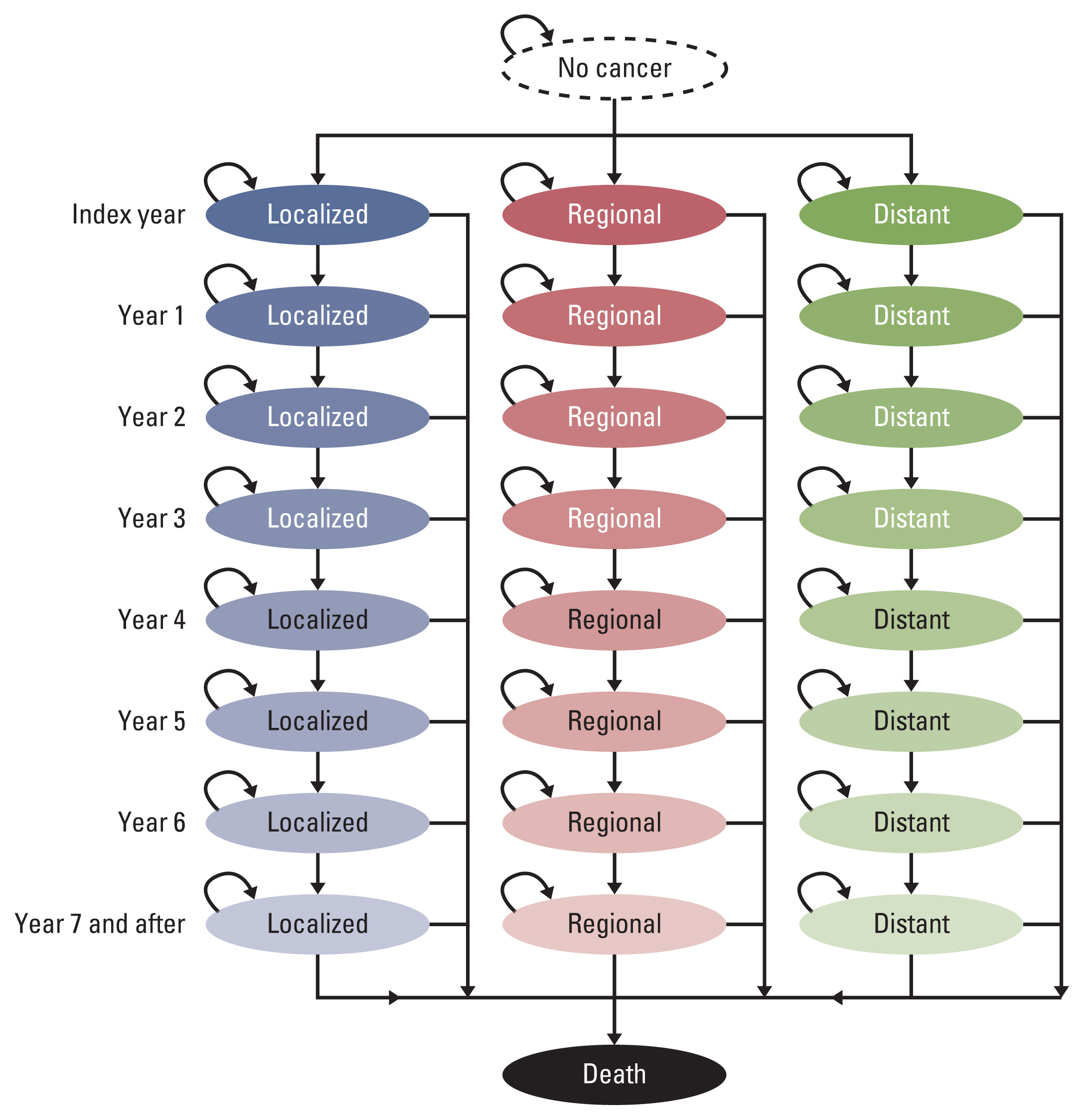
We constructed a Markov model consisting of 26 states based on the natural history of lung cancer according to the Surveillance, Epidemiology, and End Results summary stage (localized, regional, distant). In the base case, people aged 55-74 years were under consideration for annual screening. Costs and quality-adjusted life years were simulated to calculate the incremental cost utility ratio. Sensitivity analyses were performed on the uncertainty associated with screening target ages, stage distribution, cost, utility, mortality, screening duration, and discount rate.
Results
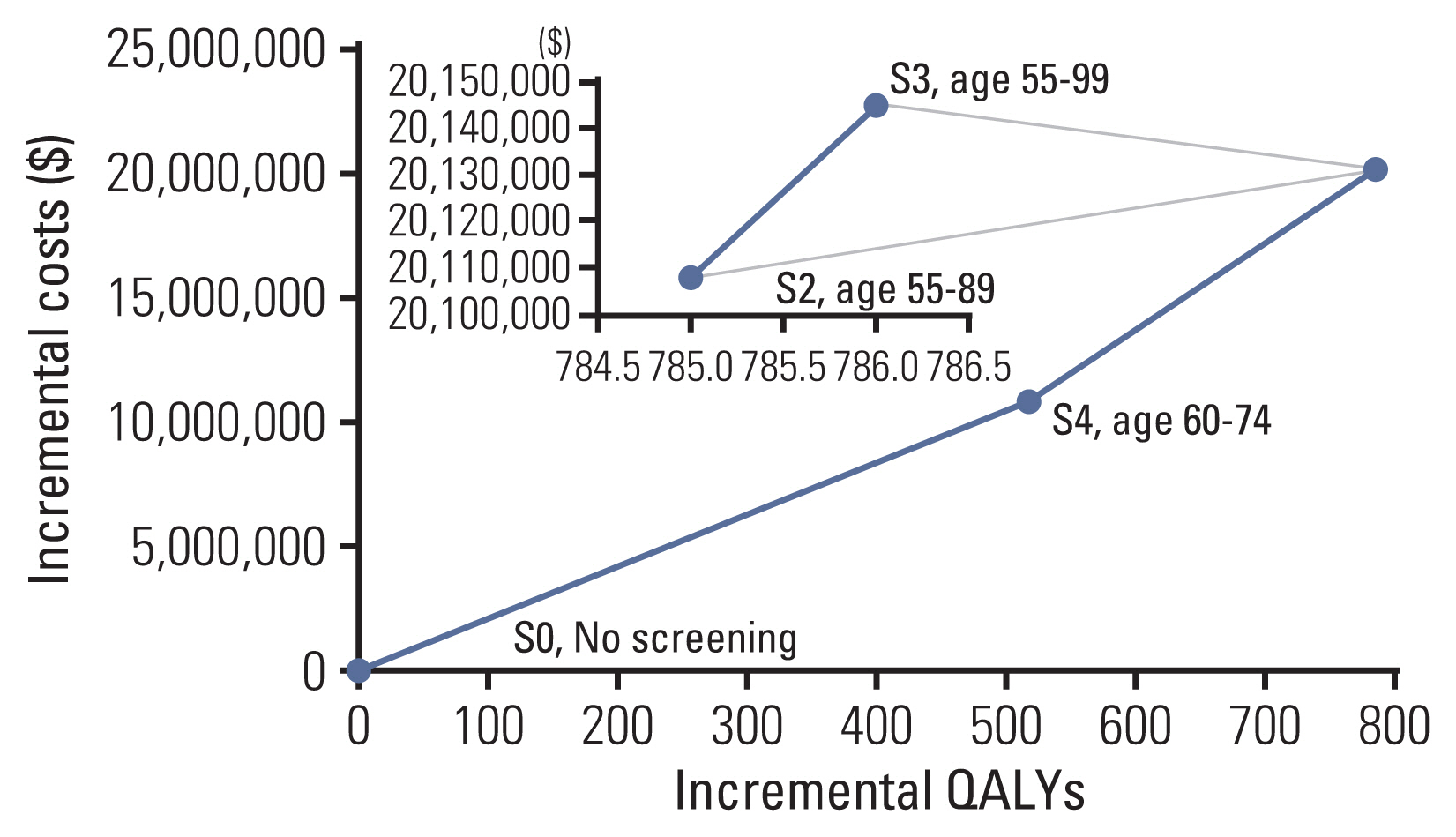
The base case (US$25,383 per quality-adjusted life year gained) was cost-effective compared to the scenario of no screening and acceptable considering a willingness-to-pay threshold of US$27,000 per quality-adjusted life years gained. In terms of the target age of screening, the age between 60 and 74 years was the most cost-effective. Lung cancer screening was still cost-effective in the sensitivity analyses on the cost for treatment, utility, mortality, screening duration, and less than 5% discount rates, although the result was sensitive to a rise in positive rates or variation of stage distribution.
Conclusion
Our results showed the cost-effectiveness of annual low-dose computed tomography screening for lung cancer in high-risk populations.
Figure
Reference
-
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–49.
Article2. Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019; 51:417–30.
Article3. Jung KW, Won YJ, Kong HJ, Lee ES. Prediction of cancer incidence and mortality in Korea, 2019. Cancer Res Treat. 2019; 51:431–7.
Article4. Organization for Economic Co-operation and Development. Daily smokers [Internet]. Paris: Organization for Economic Co-operation and Development;c2021. [cited 2021 Mar 2]. Available from: https://data.oecd.org/healthrisk/daily-smokers.htm .5. Kang MH, Park EC, Choi KS, Suh M, Jun JK, Cho E. The National Cancer Screening Program for breast cancer in the Republic of Korea: is it cost-effective? Asian Pac J Cancer Prev. 2013; 14:2059–65.
Article6. Cho E, Kang MH, Choi KS, Suh M, Jun JK, Park EC. Cost-effectiveness of Korea’s National Cervical Cancer Screening Program. Asian Pac J Cancer Prev. 2013; 14:4329–34.
Article7. Park SM, Chang YJ, Yun YH, Yoo TW, Huh BY, Kwon S. Cost-effectiveness analysis of colorectal cancer screening in Korean general population. J Korean Acad Fam Med. 2004; 25:297–306.8. Cho E, Kang MH, Choi KS, Suh M, Jun JK, Park EC. Cost-effectiveness outcomes of the national gastric cancer screening program in South Korea. Asian Pac J Cancer Prev. 2013; 14:2533–40.
Article9. Yun EH, Hong S, Her EY, Park B, Suh M, Choi KS, et al. Trends in participation rates of the National Cancer Screening Program among cancer survivors in Korea. Cancers (Basel). 2020; 13:81.
Article10. National Lung Screening Trial Research Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365:395–409.
Article11. Black WC, Gareen IF, Soneji SS, Sicks JD, Keeler EB, Aberle DR, et al. Cost-effectiveness of CT screening in the National Lung Screening Trial. N Engl J Med. 2014; 371:1793–802.
Article12. de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020; 382:503–13.
Article13. Du Y, Sidorenkov G, Heuvelmans MA, Groen HJ, Vermeulen KM, Greuter MJ, et al. Cost-effectiveness of lung cancer screening with low-dose computed tomography in heavy smokers: a microsimulation modelling study. Eur J Cancer. 2020; 135:121–9.
Article14. Griffin E, Hyde C, Long L, Varley-Campbell J, Coelho H, Robinson S, et al. Lung cancer screening by low-dose computed tomography: a cost-effectiveness analysis of alternative programmes in the UK using a newly developed natural history-based economic model. Diagn Progn Res. 2020; 4:20.
Article15. Jaine R, Kvizhinadze G, Nair N, Blakely T. Cost-effectiveness of a low-dose computed tomography screening programme for lung cancer in New Zealand. Lung Cancer. 2020; 144:99–106.
Article16. Lee J, Kim Y, Kim HY, Goo JM, Lim J, Lee CT, et al. Feasibility of implementing a national lung cancer screening program: Interim results from the Korean Lung Cancer Screening Project (K-LUCAS). Transl Lung Cancer Res. 2021; 10:723–36.
Article17. Lee J, Lim J, Kim Y, Kim HY, Goo JM, Lee CT, et al. Development of protocol for Korean Lung Cancer Screening Project (K-LUCAS) to evaluate effectiveness and feasibility to implement National Cancer Screening Program. Cancer Res Treat. 2019; 51:1285–94.
Article18. Young JL, Roffers SD, Ries LA, Fritz AG, Hurlbut AA. SEER summary staging manual - 2000: codes and coding instructions. Report No NIH Pub No. 01-4969. Bethesda, MD: National Cancer Institute;2001.19. Mahadevia PJ, Fleisher LA, Frick KD, Eng J, Goodman SN, Powe NR. Lung cancer screening with helical computed tomography in older adult smokers: a decision and cost-effectiveness analysis. JAMA. 2003; 289:313–22.
Article20. National Cancer Center. National cancer registration program [Internet]. Goyang: National Cancer Center;c2020. [cited 2021 Feb 12]. Available from: https://www.ncc.re.kr/main.ncc?uri=english/sub04_ControlPrograms02 .21. Fleurence RL, Hollenbeak CS. Rates and probabilities in economic modelling: transformation, translation and appropriate application. Pharmacoeconomics. 2007; 25:3–6.22. Korean Statistical Information Service. Complete life table [Internet]. Daejeon: Statistics Korea;c2020. [cited 2021 Aug 31]. Available from: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_1B42&conn_path=I2 .23. Gellert C, Schottker B, Brenner H. Smoking and all-cause mortality in older people: systematic review and meta-analysis. Arch Intern Med. 2012; 172:837–44.24. Kim DD, Silver MC, Kunst N, Cohen JT, Ollendorf DA, Neumann PJ. Perspective and costing in cost-effectiveness analysis, 1974–2018. Pharmacoeconomics. 2020; 38:1135–45.
Article25. Health Insurance Review and Assessment Service. Guidelines for economic evaluation of pharmaceuticals in Korea. Seoul: Health Insurance Review and Assessment Service;2011.26. Kim JH, Lee HY, Han SK, Jung HJ. Survey on the benefit coverage rate of National Health Insurance in 2006. Report No. 2007-01. Seoul: National Health Insurance;2008.27. Jung YH, Go SJ, Lee EY, Jin DR, Kim SO, Han JT, et al. A report of Korea health panel survey 2008 (1). Report No. 2009-28. Sejong: Korea Institute for Health and Social Affairs;2009.28. Lee T. Cost estimation methods in the healthcare sector. Seoul: National Evidence-based Healthcare Collaborating Agency;2011.29. Korean Statistical Information Service. 2015. currency exchange rate [Internet]. Daejeon: Statistics Korea;c2020. [cited 2021 Feb 15]. Available from: https://kosis.kr/statHtml/statHtml.do?orgId=101&tblId=DT_2KAA811_OECD&conn_path=I2 .30. Korea National Health and Nutritioin Examination Survey. Survey data [Internet]. Cheongju: Korea Disease Control and Prevention Agency;c2016. [cited 2018 Aug 3]. Available from: https://knhanes.cdc.go.kr/knhanes/sub03/sub03_02_05.do .31. Kim EJ, Ock M, Kim KP, Jung NH, Lee HJ, Kim SH, et al. Disease severity-based evaluation of utility weights for lung cancer-related health states in Korea. BMC Cancer. 2018; 18:1081.
Article32. Walters S, Maringe C, Butler J, Brierley JD, Rachet B, Coleman MP. Comparability of stage data in cancer registries in six countries: lessons from the International Cancer Benchmarking Partnership. Int J Cancer. 2013; 132:676–85.
Article33. Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, et al. The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016; 11:39–51.34. Ahn JH, Kim YH, Shin SJ, Park SY. Research on methodologies for evidence-based healthcare decision-making process in Asia. Seoul: National Evidence-Based Collaborating Agency;2012.35. Raymakers AJ, Mayo J, Lam S, FitzGerald JM, Whitehurst DG, Lynd LD. Cost-effectiveness analyses of lung cancer screening strategies using low-dose computed tomography: a systematic review. Appl Health Econ Health Policy. 2016; 14:409–18.
Article36. Song HJ, Lee EK. Evaluation of willingness to pay per quality-adjusted life year for a cure: a contingent valuation method using a scenario-based survey. Medicine (Baltimore). 2018; 97:e12453.37. American Cancer Society. Lung cancer survival rates [Internet]. Atlanta, GA: American Cancer Society;c2021. [cited 2021 Mar 1]. Available from: https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html .38. Wu FZ, Huang YL, Wu YJ, Tang EK, Wu MT, Chen CS, et al. Prognostic effect of implementation of the mass low-dose computed tomography lung cancer screening program: a hospital-based cohort study. Eur J Cancer Prev. 2020; 29:445–51.
Article39. Cataldo JK, Dubey S, Prochaska JJ. Smoking cessation: an integral part of lung cancer treatment. Oncology. 2010; 78:289–301.
Article40. Goffin JR, Flanagan WM, Miller AB, Fitzgerald NR, Memon S, Wolfson MC, et al. Cost-effectiveness of lung cancer screening in Canada. JAMA Oncol. 2015; 1:807–13.
Article41. Villanti AC, Jiang Y, Abrams DB, Pyenson BS. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS One. 2013; 8:e71379.
Article42. Kim EY, Shim YS, Kim YS, Lee SP, Ko KD, Choi WJ. Adherence to general medical checkup and cancer screening guidelines according to self-reported smoking status: Korea National Health and Nutrition Examination Survey (KNHANES) 2010–2012. PLoS One. 2019; 14:e0224224.
Article43. Korean Statistical Information Service. Cancer incidence [Internet]. Daejeon: Statistics Korea;c2021. [cited 2021 Sep 3]. Available from: https://kosis.kr/statHtml/statHtml.do?orgId=117&tblId=DT_117N_A00023&conn_path=I2 .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cost Utility Analysis of National Cancer Screening Program for Gastric Cancer in Korea: A Markov Model Analysis
- Screening for Lung Cancer
- Methodological Review of Cost Effectiveness Analysis of Cancer Screening
- Cost-Utility Analysis for Colorectal Cancer Screening According to the Initiating Age of National Cancer Screening Program in Korea
- Lung Cancer Screening: Subsequent Evidences of National Lung Screening Trial