Anat Cell Biol.
2022 Jun;55(2):179-189. 10.5115/acb.21.202.
A study on the effect of JNJ-10397049 on proliferation and differentiation of neural precursor cells
- Affiliations
-
- 1Division of Medical Biotechnology, Department of Laboratory Sciences, School of Paramedical Sciences, Shiraz University of Medical Sciences, Shiraz, Iran
- 2Diagnostic Laboratory Sciences and Technology Research Center, School of Paramedical Sciences, Shiraz University of Medical Sciences, Shiraz, Iran
- 3Neural Stem Cell Laboratory, Department of Neurosurgery, McKnight Brain Institute, University of Florida, Gainesville, FL, USA
- 4Laboratory of Basic Sciences, Mohammad Rasul Allah Research Tower, Shiraz University of Medical Sciences, Shiraz, Iran
- 5Department of Neuroscience, School of Advanced Medical Sciences and Technology, Shiraz University of Medical Sciences, Shiraz, Iran
- 6Epilepsy Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
- KMID: 2531205
- DOI: http://doi.org/10.5115/acb.21.202
Abstract
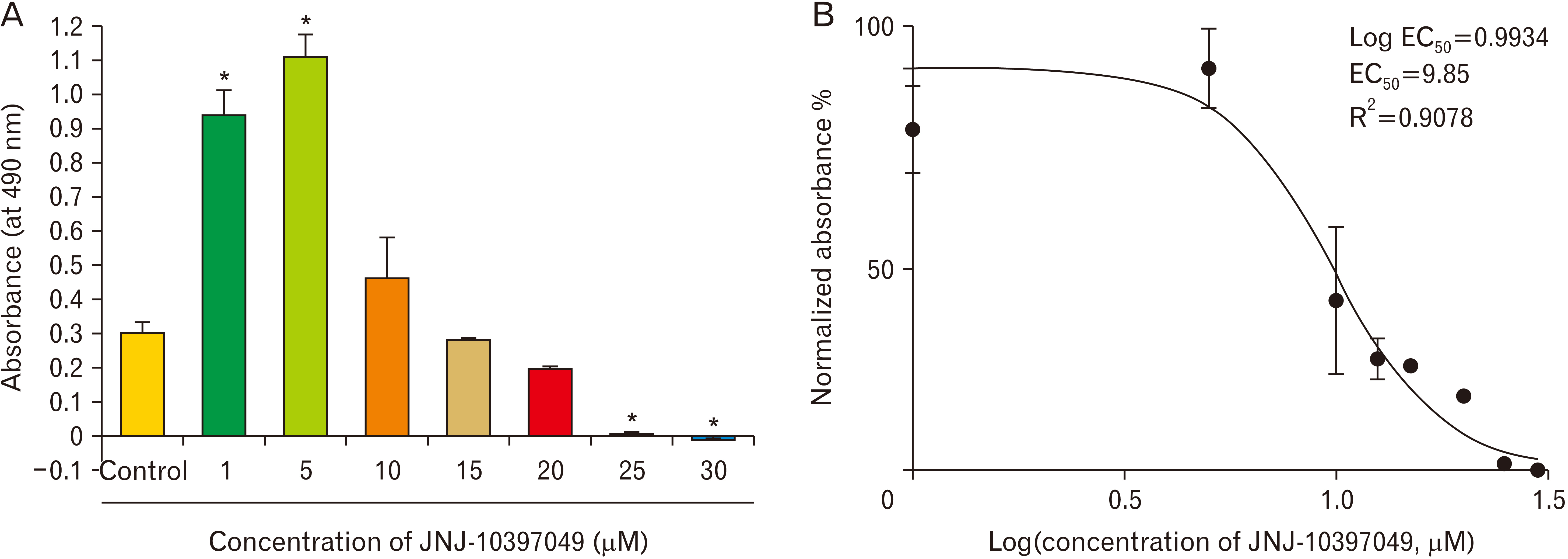
- The orexin 2 receptor plays a central role in maintaining sleep and wakefulness. Recently, it has been shown that sleep and wakefulness orchestrate the proliferation and differentiation of oligodendrocytes. Here, we explored the role of a selective orexin 2 receptor antagonist (JNJ-10397049) in proliferation and differentiation of neural progenitor cells (NPCs). We evaluated the proliferation potential of NPCs after exposure to different concentrations of JNJ-10397049 by using 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide and neurosphere assays. Moreover, the expression of differentiation markers was assessed by immunocytochemistry and real-time polymerase chain reaction. JNJ-10397049 significantly increased the proliferation of NPCs at lower concentrations. In addition, orexin 2 receptor antagonist facilitated progression of differentiation of NPCs towards oligodendroglial lineage by considerable expression of Olig2 and 2’,3’-cyclicnucleotide 3’-phosphodiesterase as well as decreased expression of nestin marker. The results open a new avenue for future investigations in which the production of more oligodendrocytes from NPCs is needed.
Figure
Reference
-
References
1. Gage FH. 2000; Mammalian neural stem cells. Science. 287:1433–8. DOI: 10.1126/science.287.5457.1433. PMID: 10688783.
Article2. Ming GL, Song H. 2011; Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 70:687–702. DOI: 10.1016/j.neuron.2011.05.001. PMID: 21609825. PMCID: PMC3106107.
Article3. Takano M, Kawabata S, Shibata S, Yasuda A, Nori S, Tsuji O, Nagoshi N, Iwanami A, Ebise H, Horiuchi K, Okano H, Nakamura M. 2017; Enhanced functional recovery from spinal cord injury in aged mice after stem cell transplantation through HGF induction. Stem Cell Reports. 8:509–18. DOI: 10.1016/j.stemcr.2017.01.013. PMID: 28216143. PMCID: PMC5355635.
Article4. Desai RA, Davies AL, Tachrount M, Kasti M, Laulund F, Golay X, Smith KJ. 2016; Cause and prevention of demyelination in a model multiple sclerosis lesion. Ann Neurol. 79:591–604. DOI: 10.1002/ana.24607. PMID: 26814844. PMCID: PMC4949637.
Article5. Haider L, Zrzavy T, Hametner S, Höftberger R, Bagnato F, Grabner G, Trattnig S, Pfeifenbring S, Brück W, Lassmann H. 2016; The topograpy of demyelination and neurodegeneration in the multiple sclerosis brain. Brain. 139(Pt 3):807–15. DOI: 10.1093/brain/awv398. PMID: 26912645. PMCID: PMC4766379.
Article6. Cohen-Adad J, El Mendili MM, Lehéricy S, Pradat PF, Blancho S, Rossignol S, Benali H. 2011; Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage. 55:1024–33. DOI: 10.1016/j.neuroimage.2010.11.089. PMID: 21232610.
Article7. Bouhrara M, Reiter DA, Bergeron CM, Zukley LM, Ferrucci L, Resnick SM, Spencer RG. 2018; Evidence of demyelination in mild cognitive impairment and dementia using a direct and specific magnetic resonance imaging measure of myelin content. Alzheimers Dement. 14:998–1004. DOI: 10.1016/j.jalz.2018.03.007. PMID: 29679574. PMCID: PMC6097903.
Article8. Mitew S, Kirkcaldie MT, Halliday GM, Shepherd CE, Vickers JC, Dickson TC. 2010; Focal demyelination in Alzheimer's disease and transgenic mouse models. Acta Neuropathol. 119:567–77. DOI: 10.1007/s00401-010-0657-2. PMID: 20198482.
Article9. Franklin RJ, Ffrench-Constant C. 2008; Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 9:839–55. DOI: 10.1038/nrn2480. PMID: 18931697.
Article10. Yue T, Xian K, Hurlock E, Xin M, Kernie SG, Parada LF, Lu QR. 2006; A critical role for dorsal progenitors in cortical myelination. J Neurosci. 26:1275–80. DOI: 10.1523/JNEUROSCI.4717-05.2006. PMID: 16436615. PMCID: PMC6674567.
Article11. Lubetzki C, Zalc B, Williams A, Stadelmann C, Stankoff B. 2020; Remyelination in multiple sclerosis: from basic science to clinical translation. Lancet Neurol. 19:678–88. DOI: 10.1016/S1474-4422(20)30140-X. PMID: 32702337.
Article12. Melchor GS, Khan T, Reger JF, Huang JK. 2019; Remyelination pharmacotherapy investigations highlight diverse mechanisms underlying multiple sclerosis progression. ACS Pharmacol Transl Sci. 2:372–86. DOI: 10.1021/acsptsci.9b00068. PMID: 32259071. PMCID: PMC7088971.
Article13. Chen Y, Zhen W, Guo T, Zhao Y, Liu A, Rubio JP, Krull D, Richardson JC, Lu H, Wang R. 2017; Histamine receptor 3 negatively regulates oligodendrocyte differentiation and remyelination. PLoS One. 12:e0189380. DOI: 10.1371/journal.pone.0189380. PMID: 29253893. PMCID: PMC5734789.
Article14. Fan LW, Bhatt A, Tien LT, Zheng B, Simpson KL, Lin RC, Cai Z, Kumar P, Pang Y. 2015; Exposure to serotonin adversely affects oligodendrocyte development and myelination in vitro. J Neurochem. 133:532–43. DOI: 10.1111/jnc.12988. PMID: 25382136. PMCID: PMC4400220.15. Ghareghani M, Sadeghi H, Zibara K, Danaei N, Azari H, Ghanbari A. 2017; Melatonin increases oligodendrocyte differentiation in cultured neural stem cells. Cell Mol Neurobiol. 37:1319–24. DOI: 10.1007/s10571-016-0450-4. PMID: 27987059.
Article16. Olivier P, Fontaine RH, Loron G, Van Steenwinckel J, Biran V, Massonneau V, Kaindl A, Dalous J, Charriaut-Marlangue C, Aigrot MS, Pansiot J, Verney C, Gressens P, Baud O. 2009; Melatonin promotes oligodendroglial maturation of injured white matter in neonatal rats. PLoS One. 4:e7128. DOI: 10.1371/journal.pone.0007128. PMID: 19771167. PMCID: PMC2742165.
Article17. Sotthibundhu A, Phansuwan-Pujito P, Govitrapong P. 2010; Melatonin increases proliferation of cultured neural stem cells obtained from adult mouse subventricular zone. J Pineal Res. 49:291–300. DOI: 10.1111/j.1600-079X.2010.00794.x. PMID: 20663047.
Article18. Sakurai T, Mieda M. 2011; Connectomics of orexin-producing neurons: interface of systems of emotion, energy homeostasis and arousal. Trends Pharmacol Sci. 32:451–62. DOI: 10.1016/j.tips.2011.03.007. PMID: 21565412.
Article19. Yin J, Babaoglu K, Brautigam CA, Clark L, Shao Z, Scheuermann TH, Harrell CM, Gotter AL, Roecker AJ, Winrow CJ, Renger JJ, Coleman PJ, Rosenbaum DM. 2016; Structure and ligand-binding mechanism of the human OX1 and OX2 orexin receptors. Nat Struct Mol Biol. 23:293–9. DOI: 10.1038/nsmb.3183. PMID: 26950369.
Article20. Ng MC. 2017; Orexin and epilepsy: potential role of REM sleep. Sleep. 40:zsw061. DOI: 10.1093/sleep/zsw061. PMID: 28364414.
Article21. Soya S, Takahashi TM, McHugh TJ, Maejima T, Herlitze S, Abe M, Sakimura K, Sakurai T. 2017; Orexin modulates behavioral fear expression through the locus coeruleus. Nat Commun. 8:1606. DOI: 10.1038/s41467-017-01782-z. PMID: 29151577. PMCID: PMC5694764. PMID: b6b7b0ef9da64f028089ee1720edac0e.
Article22. Liblau RS, Vassalli A, Seifinejad A, Tafti M. 2015; Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol. 14:318–28. DOI: 10.1016/S1474-4422(14)70218-2. PMID: 25728441.
Article23. Liguori C. 2017; Orexin and Alzheimer's disease. Curr Top Behav Neurosci. 33:305–22. DOI: 10.1007/7854_2016_50. PMID: 28012089.
Article24. Liu RJ, van den Pol AN, Aghajanian GK. 2002; Hypocretins (orexins) regulate serotonin neurons in the dorsal raphe nucleus by excitatory direct and inhibitory indirect actions. J Neurosci. 22:9453–64. DOI: 10.1523/JNEUROSCI.22-21-09453.2002. PMID: 12417670. PMCID: PMC6758063.
Article25. Lyons DJ, Hellysaz A, Ammari R, Broberger C. 2017; Hypocretin/orexin peptides excite rat neuroendocrine dopamine neurons through orexin 2 receptor-mediated activation of a mixed cation current. Sci Rep. 7:41535. DOI: 10.1038/srep41535. PMID: 28145492. PMCID: PMC5286397.
Article26. Tao R, Ma Z, McKenna JT, Thakkar MM, Winston S, Strecker RE, McCarley RW. 2006; Differential effect of orexins (hypocretins) on serotonin release in the dorsal and median raphe nuclei of freely behaving rats. Neuroscience. 141:1101–5. DOI: 10.1016/j.neuroscience.2006.05.027. PMID: 16820265.
Article27. Yamada N, Katsuura G, Tatsuno I, Asaki T, Kawahara S, Ebihara K, Saito Y, Nakao K. 2008; Orexin decreases mRNA expressions of NMDA and AMPA receptor subunits in rat primary neuron cultures. Peptides. 29:1582–7. DOI: 10.1016/j.peptides.2008.05.002. PMID: 18573570.
Article28. Yamanaka A, Tsujino N, Funahashi H, Honda K, Guan JL, Wang QP, Tominaga M, Goto K, Shioda S, Sakurai T. 2002; Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Commun. 290:1237–45. DOI: 10.1006/bbrc.2001.6318. PMID: 11811995.
Article29. Yin J, Mobarec JC, Kolb P, Rosenbaum DM. 2015; Crystal structure of the human OX2 orexin receptor bound to the insomnia drug suvorexant. Nature. 519:247–50. DOI: 10.1038/nature14035. PMID: 25533960.
Article30. Azari H, Sharififar S, Rahman M, Ansari S, Reynolds BA. 2011; Establishing embryonic mouse neural stem cell culture using the neurosphere assay. J Vis Exp. (47):2457. DOI: 10.3791/2457. PMID: 21248704. PMCID: PMC3182648.
Article31. Lassmann H, van Horssen J. 2011; The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett. 585:3715–23. DOI: 10.1016/j.febslet.2011.08.004. PMID: 21854776.
Article32. Geurts JJ, Bö L, Roosendaal SD, Hazes T, Daniëls R, Barkhof F, Witter MP, Huitinga I, van der Valk P. 2007; Extensive hippocampal demyelination in multiple sclerosis. J Neuropathol Exp Neurol. 66:819–27. DOI: 10.1097/nen.0b013e3181461f54. PMID: 17805012.
Article33. Kim LJ, Martinez D, Fiori CZ, Baronio D, Kretzmann NA, Barros HM. 2015; Hypomyelination, memory impairment, and blood-brain barrier permeability in a model of sleep apnea. Brain Res. 1597:28–36. DOI: 10.1016/j.brainres.2014.11.052. PMID: 25482664.
Article34. Pillai JA, Leverenz JB. 2017; Sleep and neurodegeneration: a critical appraisal. Chest. 151:1375–86. DOI: 10.1016/j.chest.2017.01.002. PMID: 28087304.35. Mothe AJ, Tam RY, Zahir T, Tator CH, Shoichet MS. 2013; Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials. 34:3775–83. DOI: 10.1016/j.biomaterials.2013.02.002. PMID: 23465486.
Article36. Wolswijk G. 1998; Chronic stage multiple sclerosis lesions contain a relatively quiescent population of oligodendrocyte precursor cells. J Neurosci. 18:601–9. DOI: 10.1523/JNEUROSCI.18-02-00601.1998. PMID: 9425002. PMCID: PMC6792542.
Article37. Yousefifard M, Rahimi-Movaghar V, Nasirinezhad F, Baikpour M, Safari S, Saadat S, Moghadas Jafari A, Asady H, Razavi Tousi SM, Hosseini M. 2016; Neural stem/progenitor cell transplantation for spinal cord injury treatment; a systematic review and meta-analysis. Neuroscience. 322:377–97. DOI: 10.1016/j.neuroscience.2016.02.034. PMID: 26917272.
Article38. Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. 1998; Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 92:573–85. DOI: 10.1016/S0092-8674(00)80949-6. PMID: 9527442.
Article39. Recourt K, de Boer P, Zuiker R, Luthringer R, Kent J, van der Ark P, Van Hove I, van Gerven J, Jacobs G, van Nueten L, Drevets W. 2019; The selective orexin-2 antagonist seltorexant (JNJ-42847922/MIN-202) shows antidepressant and sleep-promoting effects in patients with major depressive disorder. Transl Psychiatry. 9:216. Erratum in: Transl Psychiatry 2019;9:240. DOI: 10.1038/s41398-019-0585-4. PMID: 31578318. PMCID: PMC6775147.
Article40. Zammit G, Dauvilliers Y, Pain S, Sebök Kinter D, Mansour Y, Kunz D. 2020; Daridorexant, a new dual orexin receptor antagonist, in elderly subjects with insomnia disorder. Neurology. 94:e2222–32. DOI: 10.1212/WNL.0000000000009475. PMID: 32341187.
Article41. Elam HB, Perez SM, Donegan JJ, Lodge DJ. 2021; Orexin receptor antagonists reverse aberrant dopamine neuron activity and related behaviors in a rodent model of stress-induced psychosis. Transl Psychiatry. 11:114. DOI: 10.1038/s41398-021-01235-8. PMID: 33558469. PMCID: PMC7870676. PMID: 98cbb80306624c4e97396c531721d1e7.
Article42. Brooks S, Jacobs GE, de Boer P, Kent JM, Van Nueten L, van Amerongen G, Zuiker R, Kezic I, Luthringer R, van der Ark P, van Gerven JM, Drevets W. 2019; The selective orexin-2 receptor antagonist seltorexant improves sleep: an exploratory double-blind, placebo controlled, crossover study in antidepressant-treated major depressive disorder patients with persistent insomnia. J Psychopharmacol. 33:202–9. DOI: 10.1177/0269881118822258. PMID: 30644312.
Article43. Dubey AK, Handu SS, Mediratta PK. 2015; Suvorexant: the first orexin receptor antagonist to treat insomnia. J Pharmacol Pharmacother. 6:118–21. DOI: 10.4103/0976-500X.155496. PMID: 25969666. PMCID: PMC4419247.
Article44. Kishi T, Matsunaga S, Iwata N. 2015; Suvorexant for primary insomnia: a systematic review and meta-analysis of randomized placebo-controlled trials. PLoS One. 10:e0136910. DOI: 10.1371/journal.pone.0136910. PMID: 26317363. PMCID: PMC4552781.
Article45. Scammell TE, Winrow CJ. 2011; Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol. 51:243–66. DOI: 10.1146/annurev-pharmtox-010510-100528. PMID: 21034217. PMCID: PMC3058259.
Article46. Roth T, Black J, Cluydts R, Charef P, Cavallaro M, Kramer F, Zammit G, Walsh J. 2017; Dual orexin receptor antagonist, almorexant, in elderly patients with primary insomnia: a randomized, controlled study. Sleep. 40:zsw034. DOI: 10.1093/sleep/zsw034. PMCID: PMC5806584. PMID: 28364509.
Article47. Braley TJ, Kratz AL, Kaplish N, Chervin RD. 2016; Sleep and cognitive function in multiple sclerosis. Sleep. 39:1525–33. DOI: 10.5665/sleep.6012. PMID: 27166237. PMCID: PMC4945311.
Article48. Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, Cirelli C. 2013; Effects of sleep and wake on oligodendrocytes and their precursors. J Neurosci. 33:14288–300. DOI: 10.1523/JNEUROSCI.5102-12.2013. PMID: 24005282. PMCID: PMC3874087.
Article49. Andretic R, Franken P, Tafti M. 2008; Genetics of sleep. Annu Rev Genet. 42:361–88. DOI: 10.1146/annurev.genet.42.110807.091541. PMID: 18983259.
Article50. Bellesi M, Haswell JD, de Vivo L, Marshall W, Roseboom PH, Tononi G, Cirelli C. 2018; Myelin modifications after chronic sleep loss in adolescent mice. Sleep. 41:zsy034. DOI: 10.1093/sleep/zsy034. PMID: 29741724. PMCID: PMC5946929.
Article51. Becquet L, Abad C, Leclercq M, Miel C, Jean L, Riou G, Couvineau A, Boyer O, Tan YV. 2019; Systemic administration of orexin A ameliorates established experimental autoimmune encephalomyelitis by diminishing neuroinflammation. J Neuroinflammation. 16:64. DOI: 10.1186/s12974-019-1447-y. PMID: 30894198. PMCID: PMC6425555. PMID: 69e7f6833b604226b92795a7fa86ab95.
Article52. Fatemi I, Shamsizadeh A, Ayoobi F, Taghipour Z, Sanati MH, Roohbakhsh A, Motevalian M. 2016; Role of orexin-A in experimental autoimmune encephalomyelitis. J Neuroimmunol. 291:101–9. DOI: 10.1016/j.jneuroim.2016.01.001. PMID: 26857503.
Article53. Gencer M, Akbayır E, Şen M, Arsoy E, Yılmaz V, Bulut N, Tüzün E, Türkoğlu R. 2019; Serum orexin-A levels are associated with disease progression and motor impairment in multiple sclerosis. Neurol Sci. 40:1067–70. DOI: 10.1007/s10072-019-3708-z. PMID: 30645749.
Article54. Long KLP, Breton JM, Barraza MK, Perloff OS, Kaufer D. 2021; Hormonal regulation of oligodendrogenesis I: effects across the lifespan. Biomolecules. 11:283. DOI: 10.3390/biom11020283. PMID: 33672939. PMCID: PMC7918364. PMID: 394ed4eb50074feab425dcbd3ffb6324.
Article55. Armada-Moreira A, Ribeiro FF, Sebastião AM, Xapelli S. 2015; Neuroinflammatory modulators of oligodendrogenesis. Neuroimmunol Neuroinflammation. 2:263–73. DOI: 10.4103/2347-8659.167311.
Article56. Li H, He Y, Richardson WD, Casaccia P. 2009; Two-tier transcriptional control of oligodendrocyte differentiation. Curr Opin Neurobiol. 19:479–85. DOI: 10.1016/j.conb.2009.08.004. PMID: 19740649. PMCID: PMC2826212.
Article57. Copray S, Balasubramaniyan V, Levenga J, de Bruijn J, Liem R, Boddeke E. 2006; Olig2 overexpression induces the in vitro differentiation of neural stem cells into mature oligodendrocytes. Stem Cells. 24:1001–10. DOI: 10.1634/stemcells.2005-0239. PMID: 16253982.58. Mei F, Wang H, Liu S, Niu J, Wang L, He Y, Etxeberria A, Chan JR, Xiao L. 2013; Stage-specific deletion of Olig2 conveys opposing functions on differentiation and maturation of oligodendrocytes. J Neurosci. 33:8454–62. DOI: 10.1523/JNEUROSCI.2453-12.2013. PMID: 23658182. PMCID: PMC3865513.
Article59. Raasakka A, Myllykoski M, Laulumaa S, Lehtimäki M, Härtlein M, Moulin M, Kursula I, Kursula P. 2015; Determinants of ligand binding and catalytic activity in the myelin enzyme 2',3'-cyclic nucleotide 3'-phosphodiesterase. Sci Rep. 5:16520. DOI: 10.1038/srep16520. PMID: 26563764. PMCID: PMC4643303.
Article60. Rösener M, Muraro PA, Riethmüller A, Kalbus M, Sappler G, Thompson RJ, Lichtenfels R, Sommer N, McFarland HF, Martin R. 1997; 2',3'-cyclic nucleotide 3'-phosphodiesterase: a novel candidate autoantigen in demyelinating diseases. J Neuroimmunol. 75:28–34. DOI: 10.1016/S0165-5728(96)00230-5. PMID: 9143234.
Article61. Scherer SS, Braun PE, Grinspan J, Collarini E, Wang DY, Kamholz J. 1994; Differential regulation of the 2',3'-cyclic nucleotide 3'-phosphodiesterase gene during oligodendrocyte development. Neuron. 12:1363–75. DOI: 10.1016/0896-6273(94)90451-0. PMID: 8011341.
Article62. Raasakka A, Kursula P. 2014; The myelin membrane-associated enzyme 2',3'-cyclic nucleotide 3'-phosphodiesterase: on a highway to structure and function. Neurosci Bull. 30:956–66. DOI: 10.1007/s12264-013-1437-5. PMID: 24807122. PMCID: PMC5562554.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neural cell adhesion molecule (NCAM) promotes the differentiation of hippocampal precursor cells to a neuronal lineage, especially to a glutamatergic neural cell type
- Effects of α-asarone on Proliferation and Differentiation of Neural Progenitor Cells
- Modification of Pluripotency and Neural Crest-Related Genes' expression in Murine Skin-Derived Precursor Cells by Leukemia Inhibitory Factor (LIF)
- Roles of Gli3 in Dorsal Neural Tube
- Isolation of neural precursor cells from skeletal muscle tissues and their differentiation into neuron-like cells