Korean J Transplant.
2022 Jun;36(2):111-118. 10.4285/kjt.22.0014.
Experiences of performing ABO-incompatible kidney transplantation in Bangladesh
- Affiliations
-
- 1Department of Nephrology, Kidney Foundation Hospital and Research Institute, Dhaka, Bangladesh
- KMID: 2531134
- DOI: http://doi.org/10.4285/kjt.22.0014
Abstract
- Background
The number of end-stage renal disease (ESRD) patients is increasing in Bangladesh. Currently, living kidney donation is the only viable option for transplan-tation in Bangladesh, and it is further restricted by ABO compatibility issues. We have performed ABO-incompatible kidney transplantations (ABOi KTs) in Bangladesh since 2018. This study examines our experiences with seven cases of ABOi KT.
Methods
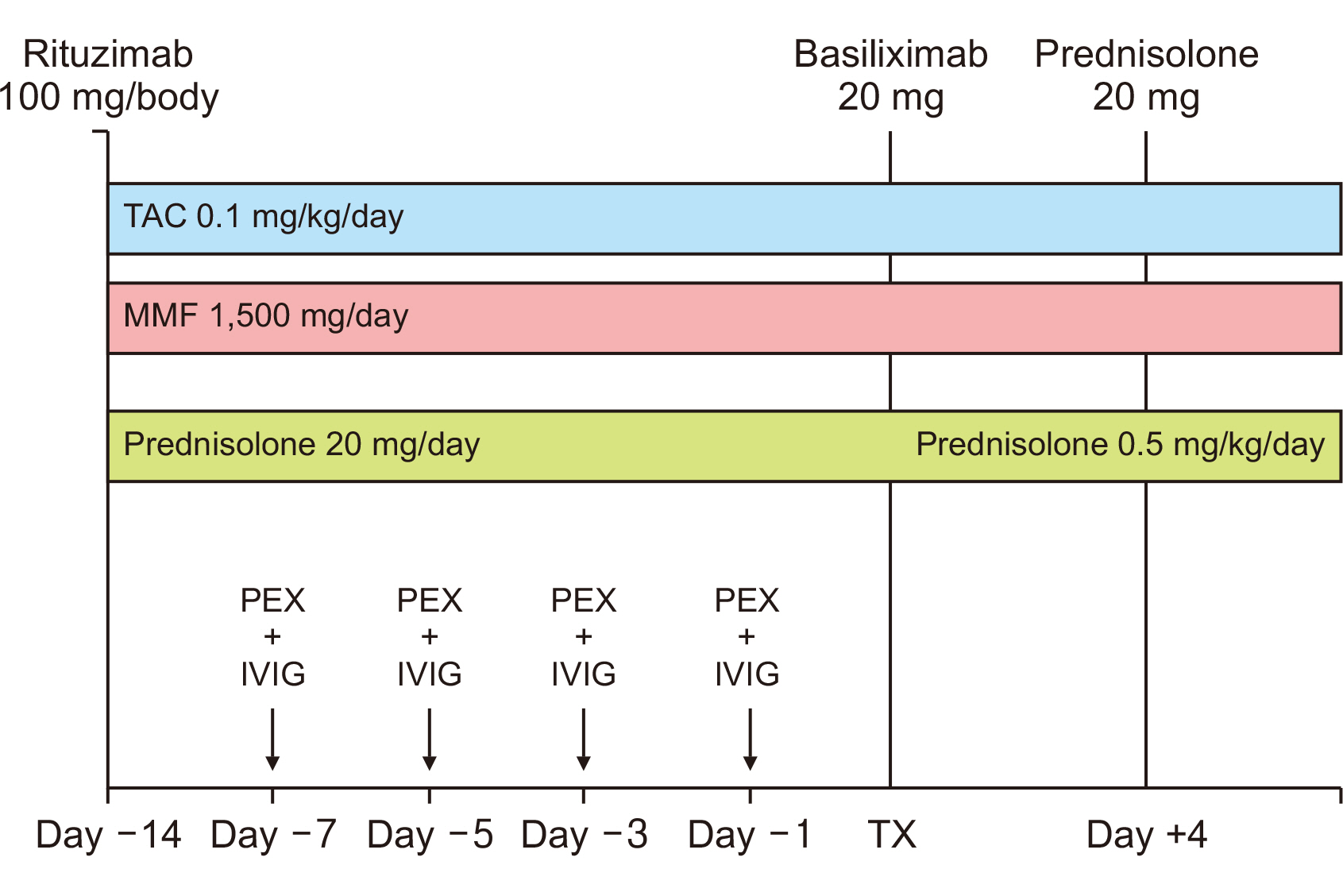
The desensitization protocol included low-dose rituximab (100 mg/body) fol-lowed by plasma exchange (PEX), which was followed by a 5-g dose of intravenous im-munoglobulin. Immunosuppression was undertaken using tacrolimus (0.1 mg/kg/day), mycophenolate mofetil (1,500 mg/day), and prednisolone (0.5 mg/kg/day). All patients received basiliximab for induction therapy.
Results
The median baseline anti-ABO antibody titer was 1:64 (range, 1:32–1:128). Transplantation was performed at a titer of ≤1:8. Our patients attended three to five PEX sessions before transplantation. Graft survival was 100% in the seven cases over a mean period of 22 months. The mean creatinine level was 204.6±47.4 µmol/L. Two patients were suspected of having developed acute rejection and received intravenous methylprednisolone, resulting in improved kidney function. One patient required post-transplant hemodialysis due to delayed graft function and subsequently improved. Infection was the most common complication experienced by ABOi KT patients. Two pa-tients developed severe cytomegalovirus pneumonia and died with functioning grafts.
Conclusions
ABOi KT in Bangladesh will substantially expand the living kidney donor pool and bring hope to a large number of ESRD patients without ABO-compatible do-nors. However, the high cost and risk of acute rejection and infection remain major concerns.
Keyword
Figure
Reference
-
1. Rashid HU, Alam MR, Khanam A, Rahman MM, Ahmed S, Mostafi M, et al. Moura-Neto JA, Divino-Filho JC, Ronco C, editors. 2021. Nephrology in Bangladesh. Nephrology Worldwide. Springer;Cham: p. 221–38. DOI: 10.1007/978-3-030-56890-0_18.2. Nepali R, Shah DS. 2019; Experience of starting ABO incompatible renal transplant in Nepal. Transpl Rep. 4:100026. DOI: 10.1016/j.tpr.2019.100026.3. Ray DS, Thukral S. 2016; Outcome of ABO-incompatible living donor renal transplantations: a single-center experience from eastern India. Transplant Proc. 48:2622–8. DOI: 10.1016/j.transproceed.2016.06.048. PMID: 27788792.4. Shirakawa H, Ishida H, Shimizu T, Omoto K, Iida S, Toki D, et al. 2011; The low dose of rituximab in ABO-incompatible kidney transplantation without a splenectomy: a single-center experience. Clin Transplant. 25:878–84. DOI: 10.1111/j.1399-0012.2010.01384.x. PMID: 21175849.5. Fuchinoue S, Ishii Y, Sawada T, Murakami T, Iwadoh K, Sannomiya A, et al. 2011; The 5-year outcome of ABO-incompatible kidney transplantation with rituximab induction. Transplantation. 91:853–7. DOI: 10.1097/TP.0b013e31820f08e8. PMID: 21297552.6. Habicht A, Bröker V, Blume C, Lorenzen J, Schiffer M, Richter N, et al. 2011; Increase of infectious complications in ABO-incompatible kidney transplant recipients: a single centre experience. Nephrol Dial Transplant. 26:4124–31. DOI: 10.1093/ndt/gfr215. PMID: 21622990.7. Lee J, Lee J, Kim S, Ju M, Joo D, Kim M, et al. 2015; Infectious complications in ABO incompatible kidney transplantation recipient according to the rituximab dose [abstract]. Am J Transplant. 15(suppl 3):C88.8. McLeod BC. 2006; Therapeutic apheresis: use of human serum albumin, fresh frozen plasma and cryosupernatant plasma in therapeutic plasma exchange. Best Pract Res Clin Haematol. 19:157–67. DOI: 10.1016/j.beha.2005.01.004. PMID: 16377548.9. Ziselman EM, Bongiovanni MB, Wurzel HA. 1984; The complications of therapeutic plasma exchange. Vox Sang. 46:270–6. DOI: 10.1111/j.1423-0410.1984.tb00086.x. PMID: 6730424.10. Yu MY, Kim YC, Lee JP, Lee H, Kim YS. 2018; Death with graft function after kidney transplantation: a single-center experience. Clin Exp Nephrol. 22:710–8. DOI: 10.1007/s10157-017-1503-9. PMID: 29159528. PMCID: PMC5956041.11. Mitra P, Hossain MG, Hossan ME, Khoda MM, Rahim MA, Hossain MJ, et al. 2018; A decade of live related donor kidney transplant: experience in a tertiary care hospital of Bangladesh. BIRDEM Med J. 8:198–202. DOI: 10.3329/birdem.v8i3.38121.12. Dong B, Wang Y, Wang G, Wang W, Zhou H, Fu Y. 2014; A retrospective study of cytomegalovirus pneumonia in renal transplant patients. Exp Ther Med. 7:1111–5. DOI: 10.3892/etm.2014.1577. PMID: 24940395. PMCID: PMC3991547.13. Chakravarti A, Kashyap B, Matlani M. 2009; Cytomegalovirus infection: an Indian perspective. Indian J Med Microbiol. 27:3–11. DOI: 10.1016/S0255-0857(21)01744-8. PMID: 19172051.14. Ullah K, Raza S, Ahmed P, Chaudhry QU, Satti TM, Ahmed S, et al. 2008; Post-transplant infections: single center experience from the developing world. Int J Infect Dis. 12:203–14. DOI: 10.1016/j.ijid.2007.06.012. PMID: 17920999.15. Gopalakrishnan V, Agarwal SK, Aggarwal S, Mahajan S, Bhowmik D, Bagchi S. 2019; Infection is the chief cause of mortality and non-death censored graft loss in the first year after renal transplantation in a resource limited population: a single centre study. Nephrology (Carlton). 24:456–63. DOI: 10.1111/nep.13401. PMID: 29761588.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ABO-Incompatible Kidney Transplantation
- Analysis of the Results of ABO-Incompatible Kidney Transplantation: In Comparison with ABO-Compatible Kidney Transplantation
- Successful Pediatric ABO-Incompatible Kidney Transplantation without Pretransplant Plasmapheresis: Report of a Case
- ABO-Incompatible Living Donor Liver Transplantation
- Case of ABO-Incompatible Living Donor Kidney Transplantation without Blood Products in a Jehovah's Witness