Blood Res.
2022 Jun;57(2):135-143. 10.5045/br.2022.2021163.
Immunoglobulin repletion during blinatumomab therapy does not reduce the rate of secondary hypogammaglobulinemia and associated infectious risk
- Affiliations
-
- 1Department of Pharmacy, The Mount Sinai Hospital, New York, NY, USA
- 2Division of Hematology/Oncology, Icahn School of Medicine at The Mount Sinai Hospital, New York, NY, USA
- KMID: 2530878
- DOI: http://doi.org/10.5045/br.2022.2021163
Abstract
- Background
Blinatumomab has demonstrated efficacy in minimal residual disease (MRD) positive and relapsed/refractory B-cell acute lymphoblastic leukemia (B-ALL) by inciting rapid and sustained B-cell depletion.
Methods
Owing to its effect on B-cells, blinatumomab is associated with a higher rate of secondary hypogammaglobulinemia compared to chemotherapy. To mitigate blinatumomab-induced hypogammaglobulinemia, patients were pre-emptively repleted with intravenous immune globulin (IVIG) during blinatumomab therapy. In this retrospective study, we compared outcomes of 23 blinatumomab treated adults with ALL. Seventeen patients routinely received IVIG and 6 patients were in the control cohort.
Results
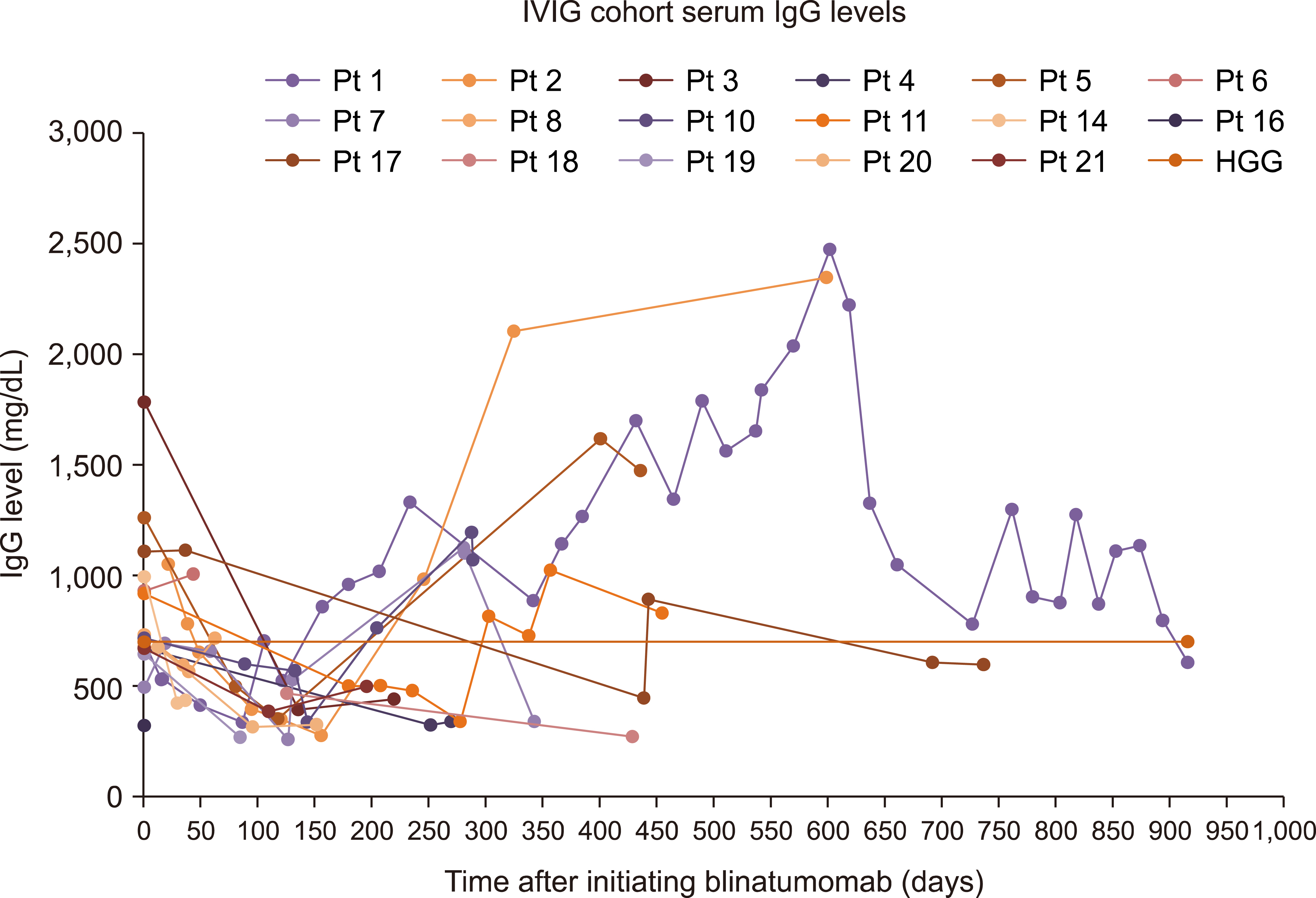
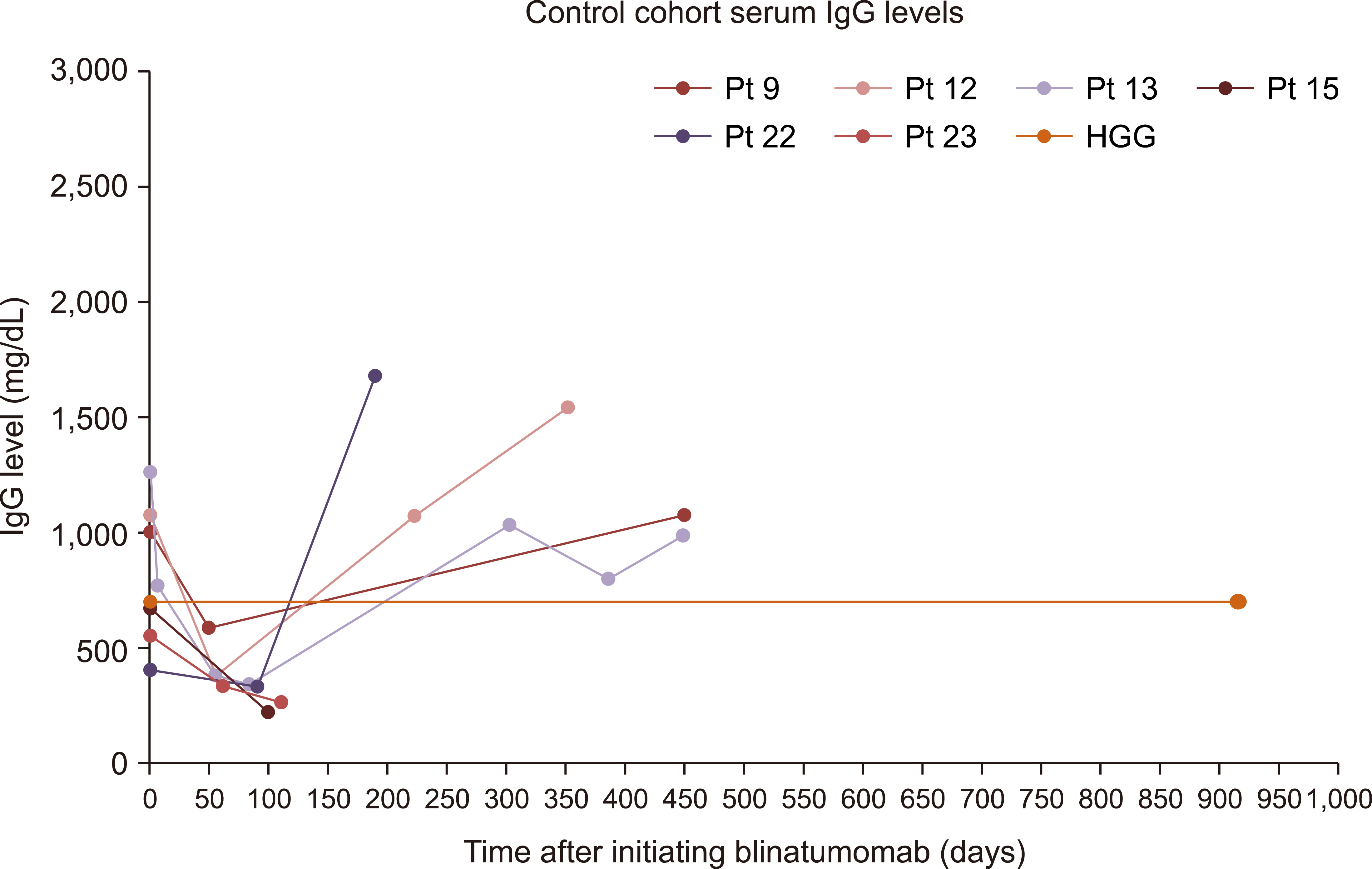
Our findings demonstrated no difference between the two cohorts in immunoglobulin G (IgG) nadir (338 mg/dL vs. 337 mg/dL, P =0.641), days to IgG nadir (120.5 vs. 85.5 days, P =0.13), infection rate (82.4% vs. 66.7%, P =0.58), infections requiring ICU admission (23.5% vs. 16.7%, P =1), and infection related mortality (17.6% vs. 16.7%, P =1).
Conclusion
Pre-emptive IVIG repletion during blinatumomab did not prevent hypogammaglobulinemia and associated infection risk.
Keyword
Figure
Reference
-
1. Berry DA, Zhou S, Higley H, et al. 2017; Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 3:e170580. DOI: 10.1001/jamaoncol.2017.0580. PMID: 28494052. PMCID: PMC5824235.2. Gökbuget N, Kneba M, Raff T, et al. 2012; Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 120:1868–76. DOI: 10.1182/blood-2011-09-377713. PMID: 22442346.
Article3. Topp MS, Gökbuget N, Zugmaier G, et al. 2012; Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 120:5185–7. DOI: 10.1182/blood-2012-07-441030. PMID: 23024237.
Article4. Jabbour EJ, Faderl S, Kantarjian HM. 2005; Adult acute lymphoblastic leukemia. Mayo Clin Proc. 80:1517–27. DOI: 10.4065/80.11.1517. PMID: 16295033.
Article5. National Comprehensive Cancer Network. 2020. Acute lymphoblastic leukemia (version 2.2020). National Comprehensive Cancer Network;Plymouth Meeting, PA: at https://www.nccn.org/professionals/physician_gls/pdf/all.pdf. Accessed November 28, 2020.6. Jabbour E, O'Brien S, Ravandi F, Kantarjian H. 2015; Monoclonal antibodies in acute lymphoblastic leukemia. Blood. 125:4010–6. DOI: 10.1182/blood-2014-08-596403. PMID: 25999456. PMCID: PMC4548495.
Article7. Raponi S, De Propris MS, Intoppa S, et al. 2011; Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: analysis of 552 cases. Leuk Lymphoma. 52:1098–107. DOI: 10.3109/10428194.2011.559668. PMID: 21348573.
Article8. 2021. Prescribing information for BlincytoⓇ (Blinatumomab). Amgen Inc;Thousand Oaks, CA: at https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/blincyto/blincyto_pi_hcp_english.pdf. Accessed March 2, 2021.9. Ribera JM, Ferrer A, Ribera J, Genescà E. 2015; Profile of blinatumomab and its potential in the treatment of relapsed/refractory acute lymphoblastic leukemia. Onco Targets Ther. 8:1567–74. DOI: 10.2147/OTT.S70524. PMID: 26170691. PMCID: PMC4485855.10. Janeway CA Jr, Travers P, Walport M, Shlomchik MJ. 2001. Immunobiology: the immune system in health and disease. 5th ed. Garland Science;New York, NY: DOI: 10.3109/10428194.2011.559668.11. Zugmaier G, Topp MS, Alekar S, et al. 2014; Long-term follow-up of serum immunoglobulin levels in blinatumomab-treated patients with minimal residual disease-positive B-precursor acute lymphoblastic leukemia. Blood Cancer J. 4:e244. DOI: 10.1038/bcj.2014.64. PMID: 25192414. PMCID: PMC4183773.
Article12. Kantarjian H, Stein A, Gökbuget N, et al. 2017; Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 376:836–47. DOI: 10.1056/NEJMoa1609783. PMID: 28249141. PMCID: PMC5881572.
Article13. Patel SY, Carbone J, Jolles S. 2019; The expanding field of secondary antibody deficiency: causes, diagnosis, and management. Front Immunol. 10:33. DOI: 10.3389/fimmu.2019.00033. PMID: 30800120. PMCID: PMC6376447.
Article14. Ueda M, Berger M, Gale RP, Lazarus HM. 2018; Immunoglobulin therapy in hematologic neoplasms and after hematopoietic cell transplantation. Blood Rev. 32:106–15. DOI: 10.1016/j.blre.2017.09.003. PMID: 28958644.
Article15. Compagno N, Malipiero G, Cinetto F, Agostini C. 2014; Immuno-globulin replacement therapy in secondary hypogammaglobulinemia. Front Immunol. 5:626. DOI: 10.3389/fimmu.2014.00626. PMID: 25538710. PMCID: PMC4259107.
Article16. Raanani P, Gafter-Gvili A, Paul M, Ben-Bassat I, Leibovici L, Shpilberg O. 2008; Immunoglobulin prophylaxis in hematological malignancies and hematopoietic stem cell transplantation. Cochrane Database Syst Rev. CD006501. DOI: 10.1002/14651858.CD006501.pub2. PMID: 18843719.
Article17. Gale RP, Chapel HM, et al. Cooperative Group for the Study of Immunoglobulin in Chronic Lymphocytic Leukemia. 1988; Intravenous immunoglobulin for the prevention of infection in chronic lymphocytic leukemia. A randomized, controlled clinical trial. N Engl J Med. 319:902–7. DOI: 10.1056/NEJM198810063191403. PMID: 2901668.18. Portell CA, Wenzell CM, Advani AS. 2013; Clinical and pharmacologic aspects of blinatumomab in the treatment of B-cell acute lymphoblastic leukemia. Clin Pharmacol. 5(Suppl 1):5–11. DOI: 10.2147/CPAA.S42689. PMID: 23671399. PMCID: PMC3650887.
Article19. Boros P, Gondolesi G, Bromberg JS. 2005; High dose intravenous immunoglobulin treatment: mechanisms of action. Liver Transpl. 11:1469–80. DOI: 10.1002/lt.20594. PMID: 16315304.
Article20. Arnold DE, Maude SL, Callahan CA, DiNofia AM, Grupp SA, Heimall JR. 2020; Subcutaneous immunoglobulin replacement following CD19-specific chimeric antigen receptor T-cell therapy for B-cell acute lymphoblastic leukemia in pediatric patients. Pediatr Blood Cancer. 67:e28092. DOI: 10.1002/pbc.28092. PMID: 31793170.
Article21. Barmettler S, Ong MS, Farmer JR, Choi H, Walter J. 2018; Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw Open. 1:e184169. DOI: 10.1001/jamanetworkopen.2018.4169. PMID: 30646343. PMCID: PMC6324375.
Article22. Lexi-Drugs. 2020. Immune globulin. Lexicomp Inc;Hudson, OH: at http://online.lexi.com. Accessed November 28, 2020.23. Zhu M, Wu B, Brandl C, et al. 2016; Blinatumomab, a bispecific T-cell engager (BiTE(®)) for CD-19 targeted cancer immunotherapy: clinical pharmacology and its implications. Clin Pharmacokinet. 55:1271–88. DOI: 10.1007/s40262-016-0405-4. PMID: 27209293.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Carpal Tunnel Syndrome in Children with Hypogammaglobulinemia: Case Report
- Blinatumomab as a Curative Therapy Option for Relapsed/Refractory Infant Acute Lymphoblastic Leukemia Post-Hematopoietic Stem Cell Transplantation - Case Report
- Stromal Keratitis in a Patient with Congenital Hypogammaglobulinemia
- Osteoarthritis of Hip in a Hypogammaglobulinemia Patient
- Membranous Nephropathy in a 13-Year-Old Boy with Common Variable Immunodeficiency