Clin Endosc.
2022 May;55(3):355-364. 10.5946/ce.2021.228.
Does computer-aided diagnostic endoscopy improve the detection of commonly missed polyps? A meta-analysis
- Affiliations
-
- 1Institute of Global Health Innovation, Imperial College, London, UK
- 2Department of Surgery and Cancer, Imperial College NHS Healthcare Trust, London, UK
- 3Department of Gastroenterology, Chelsea and Westminster NHS Healthcare Trust, London, UK
- KMID: 2529955
- DOI: http://doi.org/10.5946/ce.2021.228
Abstract
- Background/Aims
Colonoscopy is the gold standard diagnostic method for colorectal neoplasia, allowing detection and resection of adenomatous polyps; however, significant proportions of adenomas are missed. Computer-aided detection (CADe) systems in endoscopy are currently available to help identify lesions. Diminutive (≤5 mm) and nonpedunculated polyps are most commonly missed. This meta-analysis aimed to assess whether CADe systems can improve the real-time detection of these commonly missed lesions.
Methods
A comprehensive literature search was performed. Randomized controlled trials evaluating CADe systems categorized by morphology and lesion size were included. The mean number of polyps and adenomas per patient was derived. Independent proportions and their differences were calculated using DerSimonian and Laird random-effects modeling.
Results
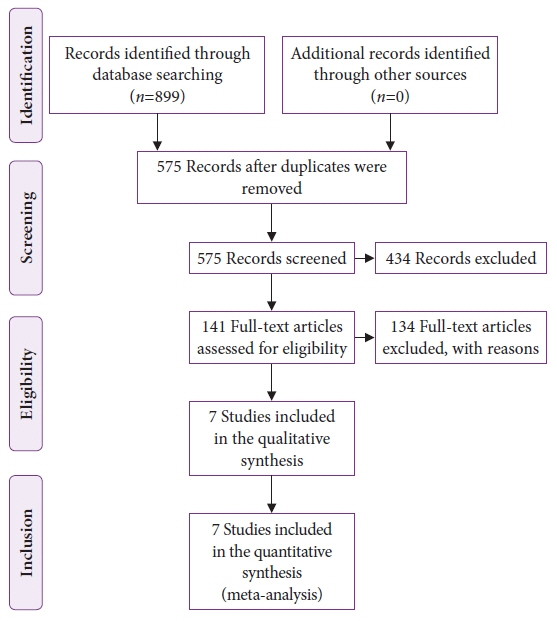
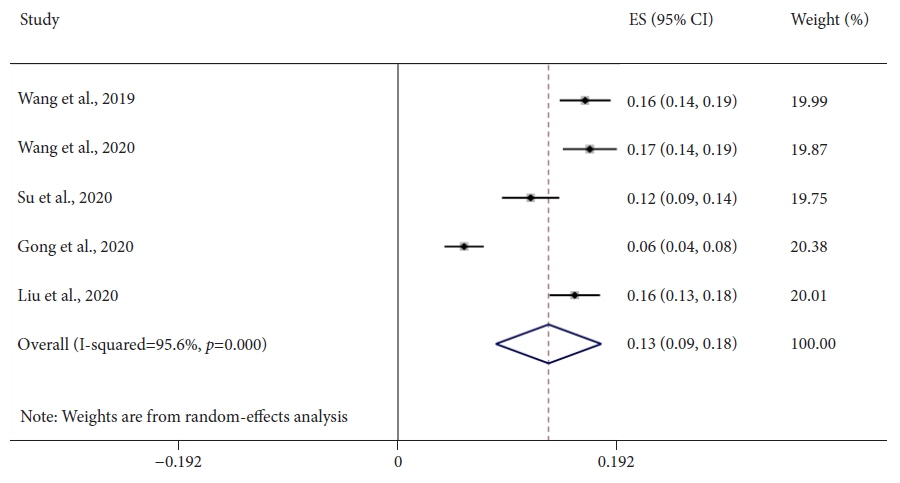
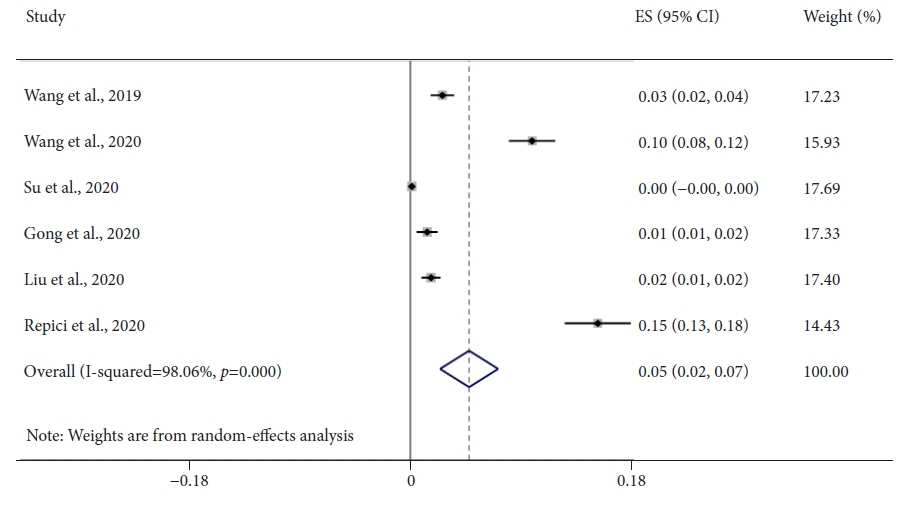
Seven studies, including 2,595 CADe-assisted colonoscopies and 2,622 conventional colonoscopies, were analyzed. CADe-assisted colonoscopy demonstrated an 80% increase in the mean number of diminutive adenomas detected per patient compared with conventional colonoscopy (0.31 vs. 0.17; effect size, 0.13; 95% confidence interval [CI], 0.09–0.18); it also demonstrated a 91.7% increase in the mean number of nonpedunculated adenomas detected per patient (0.32 vs. 0.19; effect size, 0.05; 95% CI, 0.02–0.07).
Conclusions
CADe-assisted endoscopy significantly improved the detection of most commonly missed adenomas. Although this method is a potentially exciting technology, limitations still apply to current data, prompting the need for further real-time studies.
Figure
Cited by 1 articles
-
As how artificial intelligence is revolutionizing endoscopy
Jean-Francois Rey
Clin Endosc. 2024;57(3):302-308. doi: 10.5946/ce.2023.230.
Reference
-
1. Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017; 66:683–691.2. Centelles JJ. General aspects of colorectal cancer. ISRN Oncol. 2012; 2012:1–19.3. Leslie A, Carey FA, Pratt NR, et al. The colorectal adenoma-carcinoma sequence. Br J Surg. 2002; 89:845–860.4. Brenner H, Chang-Claude J, Jansen L, et al. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology. 2014; 146:709–717.5. Kahi CJ, Myers LJ, Slaven JE, et al. Lower endoscopy reduces colorectal cancer incidence in older individuals. Gastroenterology. 2014; 146:718–725.e3.6. Zhao S, Wang S, Pan P, et al. Magnitude, risk factors, and factors associated with adenoma miss rate of tandem colonoscopy: a systematic review and meta-analysis. Gastroenterology. 2019; 156:1661–1674.e11.7. Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer. N Engl J Med. 2014; 370:1298–1306.8. Rutter MD, Beintaris I, Valori R, et al. World Endoscopy Organization consensus statements on post-colonoscopy and post-imaging colorectal cancer. Gastroenterology. 2018; 155:909–925.e3.9. Anderson R, Burr NE, Valori R. Causes of post-colonoscopy colorectal cancers based on World Endoscopy Organization system of analysis. Gastroenterology. 2020; 158:1287–1299.e2.10. Sivananthan A, Glover B, Patel K, et al. The evolution of lower gastrointestinal endoscopy; where are we now. Ther Adv Gastrointest Endosc. 2020; 13:2631774520979591.11. Harlow C, Sivananthan A, Ayaru L, et al. Endoscopic submucosal dissection: an update on tools and accessories. Ther Adv Gastrointest Endosc. 2020; 13:2631774520957220.12. Hassan C, Spadaccini M, Iannone A, et al. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 2021; 93:77–85.e6.13. Barua I, Vinsard DG, Jodal HC, et al. Artificial intelligence for polyp detection during colonoscopy: a systematic review and meta-analysis. Endoscopy. 2021; 53:277–284.14. Nazarian S, Glover B, Ashrafian H, et al. Diagnostic accuracy of artificial intelligence and computer-aided diagnosis for the detection and characterization of colorectal polyps: systematic review and meta-analysis. J Med Internet Res. 2021; 23:e27370.15. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6:e1000097.16. Ng S, Sreenivasan AK, Pecoriello J, et al. Polyp detection rate correlates strongly with adenoma detection rate in trainee endoscopists. Dig Dis Sci. 2020; 65:2229–2233.17. Delavari A, Salimzadeh H, Bishehsari F, et al. Mean polyp per patient is an accurate and readily obtainable surrogate for adenoma detection rate: results from an opportunistic screening colonoscopy program. Middle East J Dig Dis. 2015; 7:214–219.18. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996; 17:1–12.19. Wang P, Berzin TM, Glissen Brown JR, et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019; 68:1813–1819.20. Luo Y, Zhang Y, Liu M, et al. Artificial intelligence-assisted colonoscopy for detection of colon polyps: a prospective, randomized cohort study. J Gastrointest Surg. 2021; 25:2011–2018.21. Su JR, Li Z, Shao XJ, et al. Impact of a real-time automatic quality control system on colorectal polyp and adenoma detection: a prospective randomized controlled study (with videos). Gastrointest Endosc. 2020; 91:415–424.e4.22. Gong D, Wu L, Zhang J, et al. Detection of colorectal adenomas with a real-time computer-aided system (ENDOANGEL): a randomised controlled study. Lancet Gastroenterol Hepatol. 2020; 5:352–361.23. Liu WN, Zhang YY, Bian XQ, et al. Study on detection rate of polyps and adenomas in artificial-intelligence-aided colonoscopy. Saudi J Gastroenterol. 2020; 26:13–19.24. Repici A, Badalamenti M, Maselli R, et al. Efficacy of real-time computer-aided detection of colorectal neoplasia in a randomized trial. Gastroenterology. 2020; 159:512–520.e7.25. Wang P, Liu X, Berzin TM, et al. Effect of a deep-learning computer-aided detection system on adenoma detection during colonoscopy (CADe-DB trial): a double-blind randomised study. Lancet Gastroenterol Hepatol. 2020; 5:343–351.26. Zachariah R, Samarasena J, Luba D, et al. Prediction of polyp pathology using convolutional neural networks achieves “resect and discard” thresholds. Am J Gastroenterol. 2020; 115:138–144.27. Rodriguez-Diaz E, Baffy G, Singh SK. Development of computer-assisted real time histology of colorectal polyps based on the nice criteria using nearfocus narrow-band imaging. Gastroenterology. 2017; 152(5 Suppl 1):S81.28. James P, Hegagi M, Hegagi M, et al. Variable endoscopist performance in proximal and distal adenoma detection during colonoscopy: a retrospective cohort study. BMC Gastroenterol. 2018; 18:73.29. Vleugels JL, Hassan C, Senore C, et al. Diminutive polyps with advanced histologic features do not increase risk for metachronous advanced colon neoplasia. Gastroenterology. 2019; 156:623–634.e3.30. Kim JY, Kim TJ, Baek SY, et al. Risk of metachronous advanced neoplasia in patients with multiple diminutive adenomas. Am J Gastroenterol. 2018; 113:1855–1861.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- As how artificial intelligence is revolutionizing endoscopy
- Digital Mammography
- Clinical Study on Duodenal Polyps Prevalence Submitted to Upper Gaatrointestinal Endoscopy
- Gastrointestinal polyp detection in endoscopic images using an improved feature extraction method
- Computer-Aided Diagnosis in Chest CT