Korean Circ J.
2022 May;52(5):354-364. 10.4070/kcj.2021.0198.
Osstem Cardiotec Centum Stent Versus Xience Alpine Stent for De Novo Coronary Artery Lesion: A Multicenter, Randomized, Parallel-Designed, Single Blind Test
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 2Division of Cardiology, Department of Internal Medicine, Keimyung University Dongsan Medical Center, Daegu, Korea
- 3Division of Cardiology, Department of Internal Medicine, Kangdong Sacred Heart Hospital, Seoul, Korea
- 4Division of Cardiology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea
- 5Division of Cardiology, Department of Internal Medicine, Korea University Anam Hospital, Seoul, Korea
- 6Division of Cardiology, Department of Internal Medicine, The Catholic University of Korea Seoul St. Mary’s Hospital, Seoul, Korea
- KMID: 2529866
- DOI: http://doi.org/10.4070/kcj.2021.0198
Abstract
- Background and objectives
To compare the safety and efficacy of a new everolimus-eluting stent with an abluminal-coated biodegradable polymer (Osstem Cardiotec Centum) with those of the Xience Alpine stent (Xience).
Methods
This randomized, prospective, multicenter, parallel-designed, single-blind trial was conducted among patients with myocardial ischemia undergoing percutaneous coronary intervention (PCI) from 21st September 2018 until 3rd July 2020. The primary efficacy endpoint was in-segment late lumen loss (LLL) at 270 days after the procedure and the primary safety endpoints were major adverse cardiac events (MACE), composite of cardiac death, myocardial infarction, and target lesion revascularization.
Results
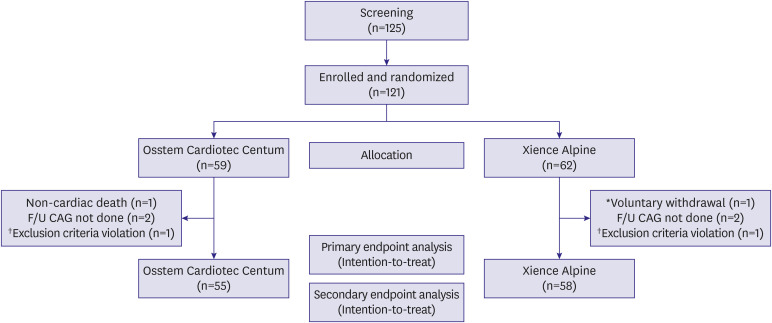
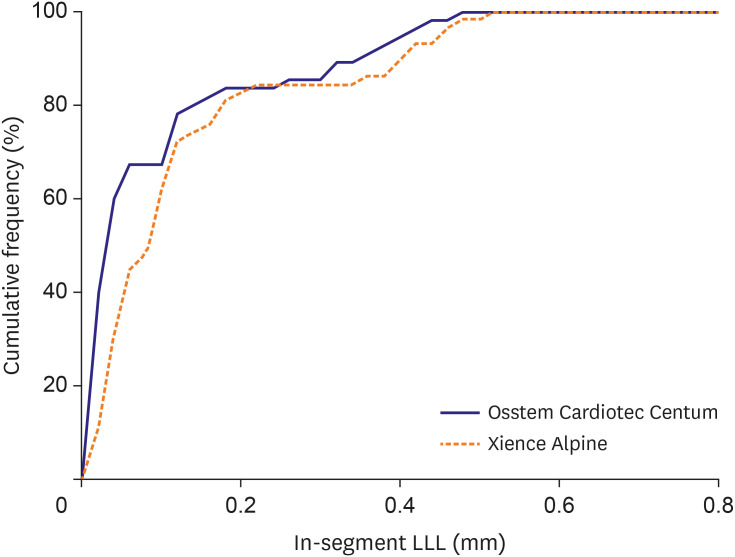
We enrolled 121 patients and analyzed 113 patients who finished 270 days of followup for the primary efficacy endpoint. The mean age of the participants was 66.8 years. As for the primary efficacy endpoint, LLL of the Osstem Cardiotec Centum group was 0.09±0.13 mm and that of the Xience group was 0.12±0.14 mm (upper limit of 1-sided 95% confidence interval, 0.02; p for non-inferiority, 0.0084). This result demonstrates the non-inferiority of the Osstem Cardiotec Centum. As for the primary safety endpoint, MACE occurred in one patient (1.59% of the Xience group). Meanwhile, no MACE occurred in the Osstem Cardiotec Centum group.
Conclusions
The Osstem Cardiotec Centum is non-inferior to the Xience Alpine ® stent and is confirmed to be safe. It could be safely and effectively applied to patients with coronary artery disease undergoing PCI.
Keyword
Figure
Cited by 1 articles
-
State-of-the-Art Stent Technology to Minimize the Risk of Stent Thrombosis and In-Stent Restenosis: Abluminal-Coated Biodegradable Polymer Drug-Eluting Stent
Dong Oh Kang, Cheol Ung Choi
Korean Circ J. 2022;52(5):365-367. doi: 10.4070/kcj.2022.0017.
Reference
-
1. Stefanini GG, Holmes DR Jr. Drug-eluting coronary-artery stents. N Engl J Med. 2013; 368:254–265. PMID: 23323902.
Article2. Kang SH, Gogas BD, Jeon KH, et al. Long-term safety of bioresorbable scaffolds: insights from a network meta-analysis including 91 trials. EuroIntervention. 2018; 13:1904–1913. PMID: 29278353.
Article3. Zanchin C, Ueki Y, Zanchin T, et al. Everolimus-eluting biodegradable polymer versus everolimus-eluting durable polymer stent for coronary revascularization in routine clinical practice. JACC Cardiovasc Interv. 2019; 12:1665–1675. PMID: 31422088.
Article4. Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med. 2010; 363:136–146. PMID: 20554978.
Article5. Stone GW, Sabik JF, Serruys PW, et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med. 2016; 375:2223–2235. PMID: 27797291.
Article6. Räber L, Magro M, Stefanini GG, et al. Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation. 2012; 125:1110–1121. PMID: 22302840.
Article7. Stone GW, Midei M, Newman W, et al. Comparison of an everolimus-eluting stent and a paclitaxel-eluting stent in patients with coronary artery disease: a randomized trial. JAMA. 2008; 299:1903–1913. PMID: 18430909.
Article8. Meredith IT, Whitbourn R, Scott D, et al. PLATINUM QCA: a prospective, multicentre study assessing clinical, angiographic, and intravascular ultrasound outcomes with the novel platinum chromium thin-strut PROMUS Element everolimus-eluting stent in de novo coronary stenoses. EuroIntervention. 2011; 7:84–90. PMID: 21550907.
Article9. Gao RL, Xu B, Lansky AJ, et al. A randomised comparison of a novel abluminal groove-filled biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: clinical and angiographic follow-up of the TARGET I trial. EuroIntervention. 2013; 9:75–83. PMID: 23685298.
Article10. Windecker S, Haude M, Neumann FJ, et al. Comparison of a novel biodegradable polymer sirolimus-eluting stent with a durable polymer everolimus-eluting stent: results of the randomized BIOFLOW-II trial. Circ Cardiovasc Interv. 2015; 8:e001441. PMID: 25634905.
Article11. Guagliumi G, Sirbu V, Musumeci G, et al. Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc Interv. 2012; 5:12–20. PMID: 22230145.
Article12. Mori M, Sakata K, Nakanishi C, et al. Early endothelialization associated with a biolimus A9 bioresorbable polymer stent in a porcine coronary model. Heart Vessels. 2017; 32:1244–1252. PMID: 28516211.
Article13. Puranik AS, Dawson ER, Peppas NA. Recent advances in drug eluting stents. Int J Pharm. 2013; 441:665–679. PMID: 23117022.
Article14. von Birgelen C, Kok MM, van der Heijden LC, et al. Very thin strut biodegradable polymer everolimus-eluting and sirolimus-eluting stents versus durable polymer zotarolimus-eluting stents in allcomers with coronary artery disease (BIO-RESORT): a three-arm, randomised, non-inferiority trial. Lancet. 2016; 388:2607–2617. PMID: 27806902.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preclinical Evaluation of a Novel Polymer-free Everolimus-eluting Stent in a Mid-term Porcine Coronary Restenosis Model
- Drug-Coated Balloons for De Novo Coronary Artery Lesions: A Meta-Analysis of Randomized Clinical Trials
- Treatment of Stent Dislodgement Complicated by Coronary Artery Dissection using Parallel Wire Technique and Small Balloon
- A Prospective, Randomized Comparison of Clinical Outcomes of the CrossFlex and NIR Stents in Coronary Intervention
- Comparison of Drug-Eluting Balloon Followed by Bare Metal Stent with Drug-Eluting Stent for Treatment of de Novo Lesions: Randomized, Controlled, Single-Center Clinical Trial