J Korean Med Sci.
2022 May;37(19):e135. 10.3346/jkms.2022.37.e135.
Strong SARS-CoV-2 Antibody Response After Booster Dose of BNT162b2 mRNA Vaccines in Uninfected Healthcare Workers
- Affiliations
-
- 1Department of Laboratory Medicine, Seoul National University Hospital, Seoul, Korea
- 2Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea
- 3Department of Laboratory Medicine, Seoul Metropolitan Government-Seoul National University Boramae Medical Center, Seoul, Korea
- 4Seegene Medical Foundation, Seoul, Korea
- KMID: 2529768
- DOI: http://doi.org/10.3346/jkms.2022.37.e135
Abstract
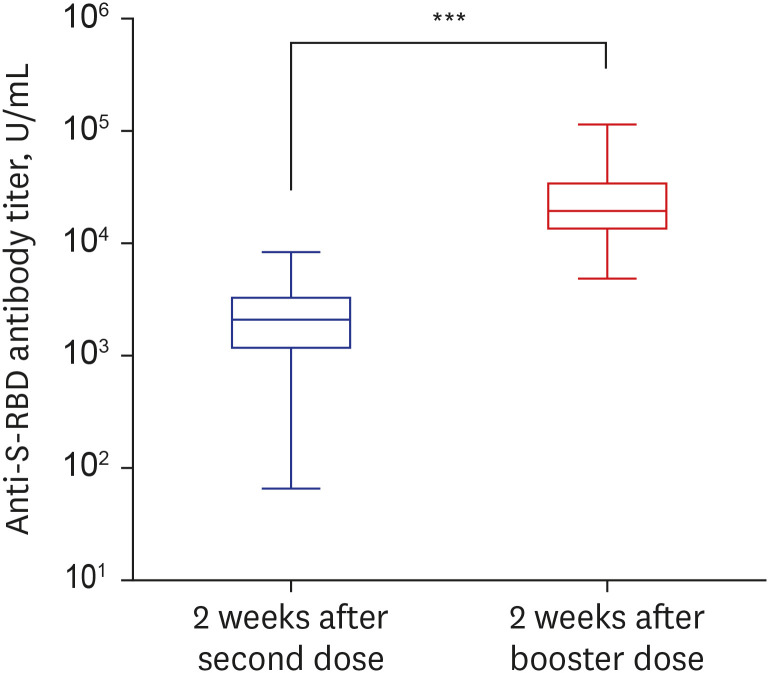
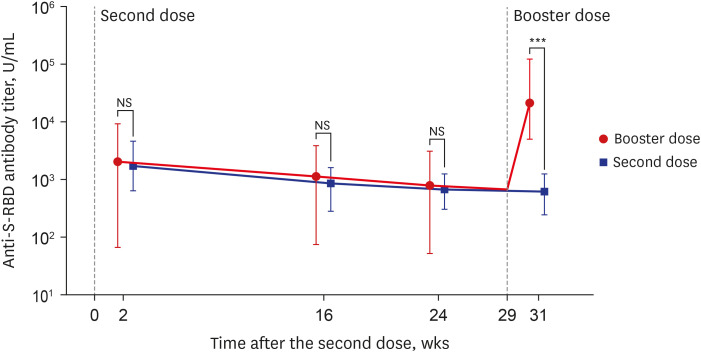
- Despite strict guidelines for coronavirus disease 2019 (COVID-19), South Korea is facing its fourth pandemic wave. In this study, by using an automated electrochemiluminescence immunoassay assay, we tracked anti-spike protein receptor-binding domain (anti-S-RBD) antibody titer from the second dose to 2 weeks after the booster dose vaccination. After the second dose, 234 participants had their anti-S-RBD antibody titers decrease over time. We also showed the booster dose (the third dose) increased antibody titer by average 14 (min–max, 2–255)-fold higher compared to the second dose among the 211-booster group participants, therefore, the booster dose could be recommended for low responders to the second dose. Our findings showed a distinct humoral response after booster doses of BNT162b2 mRNA vaccines and may provide further evidence of booster vaccination efficacy. These data will also be helpful in vaccination policy decisions that determine the need for the booster dose.
Figure
Cited by 2 articles
-
Multi-Faceted Analysis of COVID-19 Epidemic in Korea Considering Omicron Variant: Mathematical Modeling-Based Study
Youngsuk Ko, Victoria May Mendoza, Renier Mendoza, Yubin Seo, Jacob Lee, Jonggul Lee, Donghyok Kwon, Eunok Jung
J Korean Med Sci. 2022;37(26):e209. doi: 10.3346/jkms.2022.37.e209.Eight-Month Follow-up After the Third Dose of BNT162b2 Vaccine in Healthcare Workers: The Question of a Fourth Dose
Sung Hee Lim, Seong Hyeok Choi, Ji Youn Kim, Bora Kim, Han Jo Kim, Se Hyung Kim, Chan Kyu Kim, Seong Kyu Park, Jina Yun
J Korean Med Sci. 2023;38(18):e139. doi: 10.3346/jkms.2023.38.e139.
Reference
-
1. World Health Organization. The coronavirus (COVID-19) dashboard. Accessed December 5, 2021. https://covid19.who.int/ .2. Ministry of Health and Welfare. Coronavirus (COVID-19), Republic of Korea. Accessed December 5, 2021. http://ncov.mohw.go.kr/index.jsp .3. Korea Disease and Control and Prevention Agency. COVID-19 vaccination. Accessed December 5, 2021. https://ncv.kdca.go.kr/ .4. Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta KD, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021; 27(12):2127–2135. PMID: 34650248.5. Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021; 385(7):585–594. PMID: 34289274.6. Thompson RN, Hill EM, Gog JR. SARS-CoV-2 incidence and vaccine escape. Lancet Infect Dis. 2021; 21(7):913–914.7. Dalle Carbonare L, Valenti MT, Bisoffi Z, Piubelli C, Pizzato M, Accordini S, et al. Serology study after BTN162b2 vaccination in participants previously infected with SARS-CoV-2 in two different waves versus naïve. Commun Med. 2021; 1:38.8. Ducloux D, Colladant M, Chabannes M, Yannaraki M, Courivaud C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021; 100(3):702–704.9. Wu K, Choi A, Koch M, Ma L, Hill A, Nunna N, et al. Preliminary analysis of safety and immunogenicity of a SARS-CoV-2 variant vaccine booster. medRxiv. May. 6. 2021; DOI: 10.1101/2021.05.05.21256716.10. Pfizer. Second quarter 2021 Earnings teleconference 2021. Accessed December 5, 2021. https://s21.q4cdn.com/317678438/files/doc_financials/2021/q2/Q2-2021-Earnings-Charts-FINAL.pdf .11. Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021; 398(10316):2093–2100. PMID: 34756184.12. Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals. Accessed December 5, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised .13. Center for Disease Control and Prevention. Grading of Recommendation, Assessment, Development, and Evaluation (GRADE): Pfizer-BioNTech, Moderna, and Janssen COVID-19 booster dose. Accessed December 5, 2021. https://www.cdc.gov/vaccines/acip/recs/grade/covid-19-booster-doses.html/ .14. Kim N, Minn D, Park S, Roh EY, Yoon JH, Park H, et al. Positivity of SARS-CoV-2 antibodies among Korean healthy healthcare workers 1 and 2 weeks after second dose of Pfizer-BioNTech vaccination. J Korean Med Sci. 2021; 36(21):e158. PMID: 34060264.15. Kim N, Shin S, Minn D, Park S, An D, Park JH, et al. SARS-CoV-2 infectivity and antibody titer reduction for 6 months after second dose of BNT162b2 mRNA vaccine in healthcare workers: a prospective cohort study. J Infect Dis. 2022; jiac035. PMID: 35104871.16. Elecsys® anti-SARS-CoV-2 S. Insert (material number 09289267190 and 09289275190). Accessed December 5. https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2-s.html .17. Higgins V, Fabros A, Kulasingam V. Quantitative measurement of anti-SARS-CoV-2 antibodies: analytical and clinical evaluation. J Clin Microbiol. 2021; 59(4):e03149-20. PMID: 33483360.18. Eyal O, Olshevsky U, Lustig S, Paran N, Halevy M, Schneider P, et al. Development of a tissue-culture-based enzyme-immunoassay method for the quantitation of anti-vaccinia-neutralizing antibodies in human sera. J Virol Methods. 2005; 130(1-2):15–21. PMID: 16024096.19. Bewley KR, Coombes NS, Gagnon L, McInroy L, Baker N, Shaik I, et al. Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays. Nat Protoc. 2021; 16(6):3114–3140. PMID: 33893470.20. Padoan A, Bonfante F, Pagliari M, Bortolami A, Negrini D, Zuin S, et al. Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity. EBioMedicine. 2020; 62:103101. PMID: 33160207.21. Grenache DG, Ye C, Bradfute SB. Correlation of SARS-CoV-2 Neutralizing Antibodies to an Automated Chemiluminescent Serological Immunoassay. J Appl Lab Med. 2021; 6(2):491–495. PMID: 33098417.22. Cristiano A, Pieri M, Sarubbi S, Pelagalli M, Calugi G, Tomassetti F, et al. Evaluation of serological anti-SARS-CoV-2 chemiluminescent immunoassays correlated to live virus neutralization test, for the detection of anti-RBD antibodies as a relevant alternative in COVID-19 large-scale neutralizing activity monitoring. Clin Immunol. 2022; 234:108918. PMID: 34971839.23. Kohmer N, Westhaus S, Rühl C, Ciesek S, Rabenau HF. Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays. J Clin Virol. 2020; 129:104480. PMID: 32505777.24. Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020; 586(7830):594–599. PMID: 32998157.25. Zhang LX, Miao SY, Qin ZH, Wu JP, Chen HY, Sun HB, et al. Preliminary analysis of B- and T-cell responses to SARS-CoV-2. Mol Diagn Ther. 2020; 24(5):601–609. PMID: 32710269.26. Barnes CO, Jette CA, Abernathy ME, Dam KA, Esswein SR, Gristick HB, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020; 588(7839):682–687. PMID: 33045718.27. Criscuolo E, Diotti RA, Strollo M, Rolla S, Ambrosi A, Locatelli M, et al. Weak correlation between antibody titers and neutralizing activity in sera from SARS-CoV-2 infected subjects. J Med Virol. 2021; 93(4):2160–2167. PMID: 33064340.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes in SARS-CoV-2 antibody titers 6 months after the booster dose of BNT162b2 COVID-19 vaccine among health care workers
- Comparison of the rapidity of SARS-CoV-2 immune responses between primary and booster vaccination for COVID-19
- Anti-SARS-CoV-2 spike antibody response to the third dose of BNT162b2 mRNA COVID-19 vaccine and associated factors in Japanese hemodialysis patients
- Humoral immune response to SARS-CoV-2 mRNA vaccines is associated with choice of vaccine and systemic adverse reactions
- SARS-CoV-2 Breakthrough Infection after mRNA-1273 Booster among CoronaVac-Vaccinated Healthcare Workers