Korean J Physiol Pharmacol.
2022 May;26(3):219-225. 10.4196/kjpp.2022.26.3.219.
Ca2+ entry through reverse Na+ /Ca2+ exchanger in NCI-H716, glucagon-like peptide-1 secreting cells
- Affiliations
-
- 1Department of Physiology, College of Medicine, Konyang University, Daejeon 35365, Korea
- 2Myunggok Medical Research Institute, Konyang University, Daejeon 35365, Korea
- KMID: 2529404
- DOI: http://doi.org/10.4196/kjpp.2022.26.3.219
Abstract
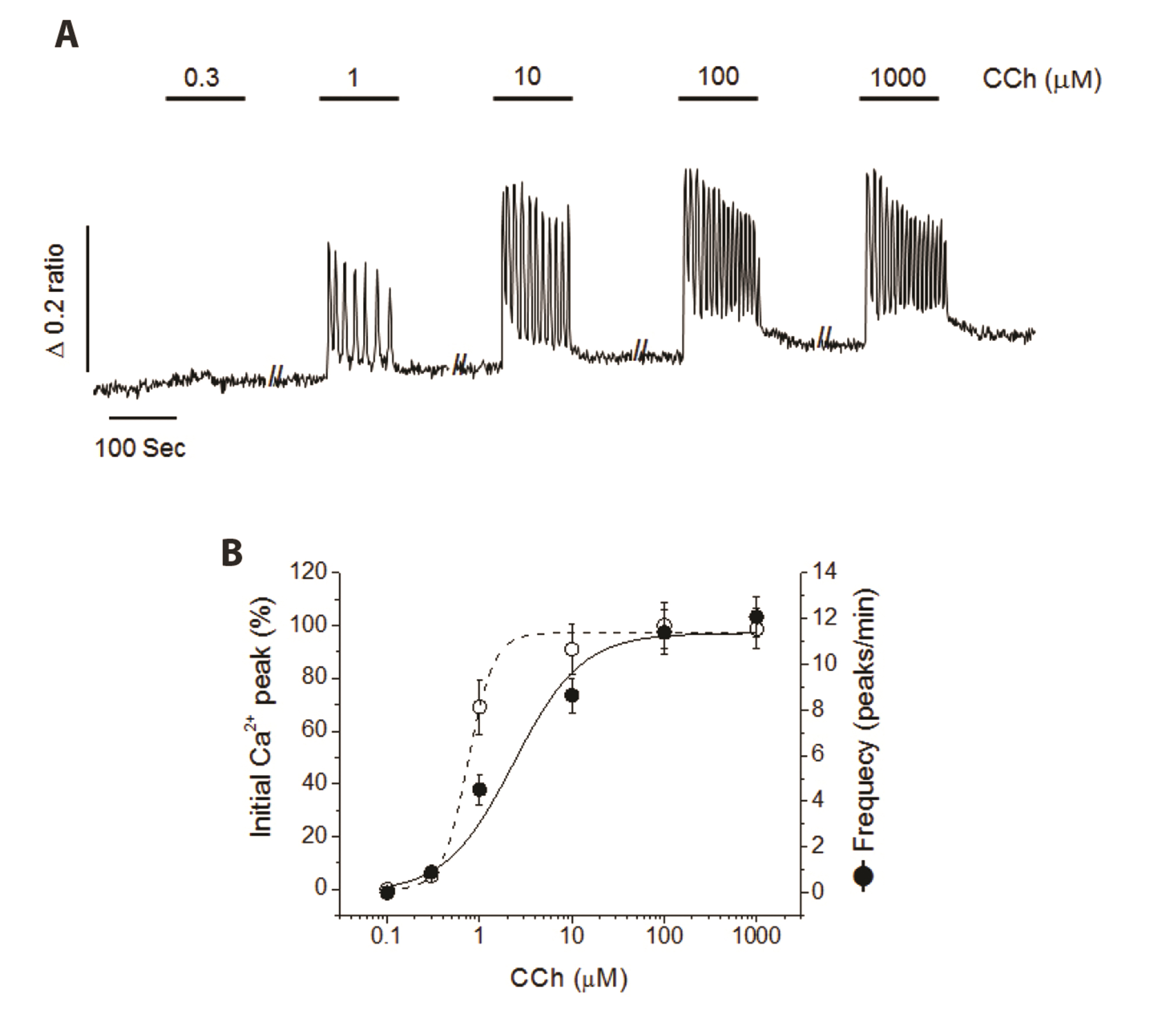
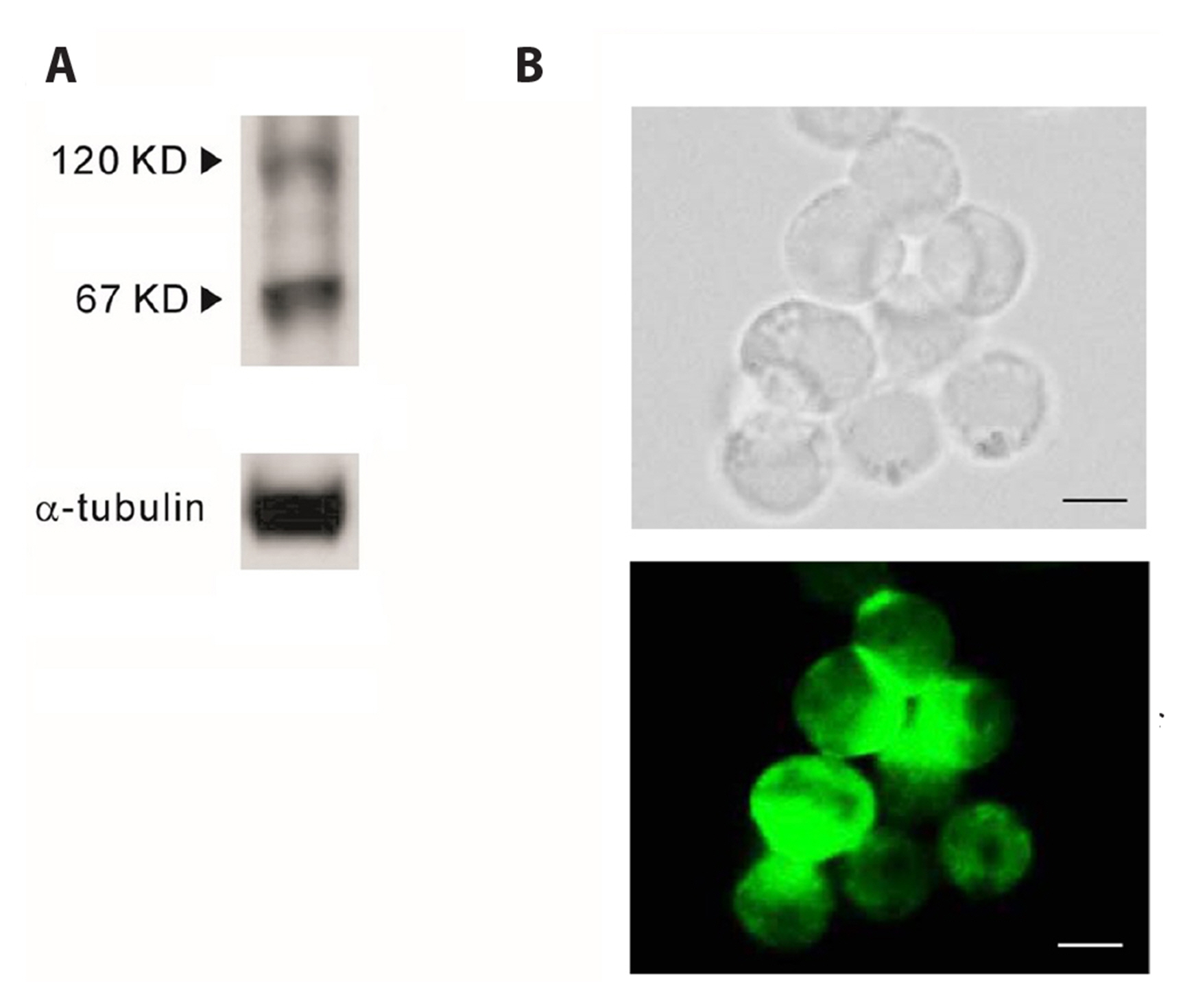
- Glucagon like peptide-1 (GLP-1) released from enteroendocine L-cells in the intestine has incretin effects due to its ability to amplify glucose-dependent insulin secretion. Promotion of an endogenous release of GLP-1 is one of therapeutic targets for type 2 diabetes mellitus. Although the secretion of GLP-1 in response to nutrient or neural stimuli can be triggered by cytosolic Ca2+ elevation, the stimulussecretion pathway is not completely understood yet. Therefore, the aim of this study was to investigate the role of reverse Na+ /Ca2+ exchanger (rNCX) in Ca2+ entry induced by muscarinic stimulation in NCI-H716 cells, a human enteroendocrine GLP-1 secreting cell line. Intracellular Ca2+ was repetitively oscillated by the perfusion of carbamylcholine (CCh), a muscarinic agonist. The oscillation of cytosolic Ca2+ was ceased by substituting extracellular Na+ with Li + or NMG + . KB-R7943, a specific rNCX blocker, completely diminished CCh-induced cytosolic Ca2+ oscillation. Type 1 Na+ /Ca2+ exchanger (NCX 1 ) proteins were expressed in NCI-H716 cells. These results suggest that rNCX might play a crucial role in Ca2+ entry induced by cholinergic stimulation in NCIH716 cells, a GLP-1 secreting cell line.
Figure
Reference
-
1. Kieffer TJ, Habener JF. 1999; The glucagon-like peptides. Endocr Rev. 20:876–913. DOI: 10.1210/edrv.20.6.0385. PMID: 10605628.
Article2. Fehmann HC, Göke R, Göke B. 1992; Glucagon-like peptide-1(7-37)/(7-36)amide is a new incretin. Mol Cell Endocrinol. 85:C39–C44. DOI: 10.1016/0303-7207(92)90118-P. PMID: 1382025.
Article3. Ma X, Guan Y, Hua X. 2014; Glucagon-like peptide 1-potentiated insulin secretion and proliferation of pancreatic β-cells. J Diabetes. 6:394–402. DOI: 10.1111/1753-0407.12161. PMID: 24725840.
Article4. Arulmozhi DK, Portha B. 2006; GLP-1 based therapy for type 2 diabetes. Eur J Pharm Sci. 28:96–108. DOI: 10.1016/j.ejps.2006.01.003. PMID: 16488579.
Article5. Abbas G, Haq QMI, Hamaed A, Al-Sibani M, Hussain H. 2020; Glucagon and glucagon-like peptide-1 receptors: promising therapeutic targets for an effective management of diabetes mellitus. Curr Pharm Des. 26:501–508. DOI: 10.2174/1381612826666200131143231. PMID: 32003684.
Article6. Kuhre RE, Wewer Albrechtsen NJ, Deacon CF, Balk-Møller E, Rehfeld JF, Reimann F, Gribble FM, Holst JJ. 2016; Peptide production and secretion in GLUTag, NCI-H716, and STC-1 cells: a comparison to native L-cells. J Mol Endocrinol. 56:201–211. DOI: 10.1530/JME-15-0293. PMID: 26819328. PMCID: PMC7212058.
Article7. Sun EW, de Fontgalland D, Rabbitt P, Hollington P, Sposato L, Due SL, Wattchow DA, Rayner CK, Deane AM, Young RL, Keating DJ. 2017; Mechanisms controlling glucose-induced GLP-1 secretion in human small intestine. Diabetes. 66:2144–2149. DOI: 10.2337/db17-0058. PMID: 28385801. PMCID: PMC5860185.
Article8. Holst JJ. 2007; The physiology of glucagon-like peptide 1. Physiol Rev. 87:1409–1439. DOI: 10.1152/physrev.00034.2006. PMID: 17928588.
Article9. Goldspink DA, Lu VB, Miedzybrodzka EL, Smith CA, Foreman RE, Billing LJ, Kay RG, Reimann F, Gribble FM. 2020; Labeling and characterization of human GLP-1-secreting L-cells in primary ileal organoid culture. Cell Rep. 31:107833. DOI: 10.1016/j.celrep.2020.107833. PMID: 32610134. PMCID: PMC7342002.
Article10. Reimer RA, Darimont C, Gremlich S, Nicolas-Métral V, Rüegg UT, Macé K. 2001; A human cellular model for studying the regulation of glucagon-like peptide-1 secretion. Endocrinology. 142:4522–4528. DOI: 10.1210/endo.142.10.8415. PMID: 11564718.
Article11. Le Nevé B, Daniel H. 2011; Selected tetrapeptides lead to a GLP-1 release from the human enteroendocrine cell line NCI-H716. Regul Pept. 167:14–20. DOI: 10.1016/j.regpep.2010.10.010. PMID: 21070823.
Article12. Suh HW, Lee KB, Kim KS, Yang HJ, Choi EK, Shin MH, Park YS, Na YC, Ahn KS, Jang YP, Um JY, Jang HJ. 2015; A bitter herbal medicine Gentiana scabra root extract stimulates glucagon-like peptide-1 secretion and regulates blood glucose in db/db mouse. J Ethnopharmacol. 172:219–226. DOI: 10.1016/j.jep.2015.06.042. PMID: 26129938.
Article13. Annunziato L, Pignataro G, Di Renzo GF. 2004; Pharmacology of brain Na+/Ca2+ exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev. 56:633–654. DOI: 10.1124/pr.56.4.5. PMID: 15602012.
Article14. Lytton J. 2007; Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J. 406:365–382. DOI: 10.1042/BJ20070619. PMID: 17716241.15. Philipson KD, Nicoll DA. 2000; Sodium-calcium exchange: a molecular perspective. Annu Rev Physiol. 62:111–133. DOI: 10.1146/annurev.physiol.62.1.111. PMID: 10845086.
Article16. Hirota S, Pertens E, Janssen LJ. 2007; The reverse mode of the Na+/Ca2+ exchanger provides a source of Ca2+ for store refilling following agonist-induced Ca2+ mobilization. Am J Physiol Lung Cell Mol Physiol. 292:L438–L447. DOI: 10.1152/ajplung.00222.2006. PMID: 17041014.
Article17. Tolhurst G, Zheng Y, Parker HE, Habib AM, Reimann F, Gribble FM. 2011; Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology. 152:405–413. DOI: 10.1210/en.2010-0956. PMID: 21209017. PMCID: PMC3140224.
Article18. Jang HJ, Kokrashvili Z, Theodorakis MJ, Carlson OD, Kim BJ, Zhou J, Kim HH, Xu X, Chan SL, Juhaszova M, Bernier M, Mosinger B, Margolskee RF, Egan JM. 2007; Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 104:15069–15074. DOI: 10.1073/pnas.0706890104. PMID: 17724330. PMCID: PMC1986614.
Article19. Kim MJ, Choi KJ, Yoon MN, Oh SH, Kim DK, Kim SH, Park HS. 2018; Hydrogen peroxide inhibits Ca2+ efflux through plasma membrane Ca2+-ATPase in mouse parotid acinar cells. Korean J Physiol Pharmacol. 22:215–223. DOI: 10.4196/kjpp.2018.22.2.215. PMID: 29520174. PMCID: PMC5840080.
Article20. Park HS, Betzenhauser MJ, Zhang Y, Yule DI. 2012; Regulation of Ca²+ release through inositol 1,4,5-trisphosphate receptors by adenine nucleotides in parotid acinar cells. Am J Physiol Gastrointest Liver Physiol. 302:G97–G104. DOI: 10.1152/ajpgi.00328.2011. PMID: 21960523. PMCID: PMC3345966.21. Petersen OH. 2017; The effects of Ca2+ buffers on cytosolic Ca2+ signalling. J Physiol. 595:3107–3108. DOI: 10.1113/JP273852. PMID: 28058721. PMCID: PMC5430207.
Article22. Philipson KD, Longoni S, Ward R. 1988; Purification of the cardiac Na+-Ca2+ exchange protein. Biochim Biophys Acta. 945:298–306. DOI: 10.1016/0005-2736(88)90492-0. PMID: 3191125.23. Anini Y, Brubaker PL. 2003; Muscarinic receptors control glucagon-like peptide 1 secretion by human endocrine L cells. Endocrinology. 144:3244–3250. DOI: 10.1210/en.2003-0143. PMID: 12810581.
Article24. Parekh AB. 2011; Decoding cytosolic Ca2+ oscillations. Trends Biochem Sci. 36:78–87. DOI: 10.1016/j.tibs.2010.07.013. PMID: 20810284.25. Smedler E, Uhlén P. 2014; Frequency decoding of calcium oscillations. Biochim Biophys Acta. 1840:964–969. DOI: 10.1016/j.bbagen.2013.11.015. PMID: 24269537.
Article26. Reimann F, Maziarz M, Flock G, Habib AM, Drucker DJ, Gribble FM. 2005; Characterization and functional role of voltage gated cation conductances in the glucagon-like peptide-1 secreting GLUTag cell line. J Physiol. 563(Pt 1):161–175. DOI: 10.1113/jphysiol.2004.076414. PMID: 15611035. PMCID: PMC1665554.
Article27. Goldspink DA, Lu VB, Billing LJ, Larraufie P, Tolhurst G, Gribble FM, Reimann F. 2018; Mechanistic insights into the detection of free fatty and bile acids by ileal glucagon-like peptide-1 secreting cells. Mol Metab. 7:90–101. DOI: 10.1016/j.molmet.2017.11.005. PMID: 29167062. PMCID: PMC5784317.
Article28. McCandless M, Nishiyama A, Petersen OH, Philpott HG. 1981; Mouse pancreatic acinar cells: voltage-clamp study of acetylcholine-evoked membrane current. J Physiol. 318:57–71. DOI: 10.1113/jphysiol.1981.sp013850. PMID: 7320904. PMCID: PMC1245477.
Article29. Morris AP, Fuller CM, Gallacher DV. 1987; Cholinergic receptors regulate a voltage-insensitive but Na+-dependent calcium influx pathway in salivary acinar cells. FEBS Lett. 211:195–199. DOI: 10.1016/0014-5793(87)81435-7. PMID: 3803598.
Article30. So I, Kim KW. 2003; Nonselective cation channels activated by the stimulation of muscarinic receptors in mammalian gastric smooth muscle. J Smooth Muscle Res. 39:231–247. DOI: 10.1540/jsmr.39.231. PMID: 15048016.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of NCX and NCKX Isoforms mRNAs in Rat Pituitary Gland
- Pancreatic alpha-Cell Dysfunction in Type 2 Diabetes: Old Kids on the Block
- Identification of a Novel Function of Extract of Gingko biloba (EGb 761®) as a Regulator of PYY Secretion and FFA4 Activation
- Glucagon-like Peptide-1 Analogue and Dipeptidyl Peptidase-IV Inhibitors
- Initiation Site of Ca2+ Entry Evoked by Endoplasmic Reticulum Ca2+ Depletion in Mouse Parotid and Pancreatic Acinar Cells