Korean J Pain.
2022 Apr;35(2):160-172. 10.3344/kjp.2022.35.2.160.
Insulin enhances neurite extension and myelination of diabetic neuropathy neurons
- Affiliations
-
- 1Singapore Institute for Neurotechnology, National University of Singapore, Singapore
- 2Department of Biotechnology, Ho Chi Minh City University of Food Industry, Ho Chi Minh City, Vietnam
- 3Department of Biomedical Engineering, National University of Singapore, Singapore
- 4Department of Biomedical Engineering, Johns Hopkins University, Baltimore, MD, USA
- KMID: 2527767
- DOI: http://doi.org/10.3344/kjp.2022.35.2.160
Abstract
- Background
The authors established an In Vitro model of diabetic neuropathy based on the culture system of primary neurons and Schwann cells (SCs) to mimic similar symptoms observed in in vivo models of this complication, such as impaired neurite extension and impaired myelination. The model was then utilized to investigate the effects of insulin on enhancing neurite extension and myelination of diabetic neurons.
Methods
SCs and primary neurons were cultured under conditions mimicking hyperglycemia prepared by adding glucose to the basal culture medium. In a single culture, the proliferation and maturation of SCs and the neurite extension of neurons were evaluated. In a co-culture, the percentage of myelination of diabetic neurons was investigated. Insulin at different concentrations was supplemented to culture media to examine its effects on neurite extension and myelination.
Results
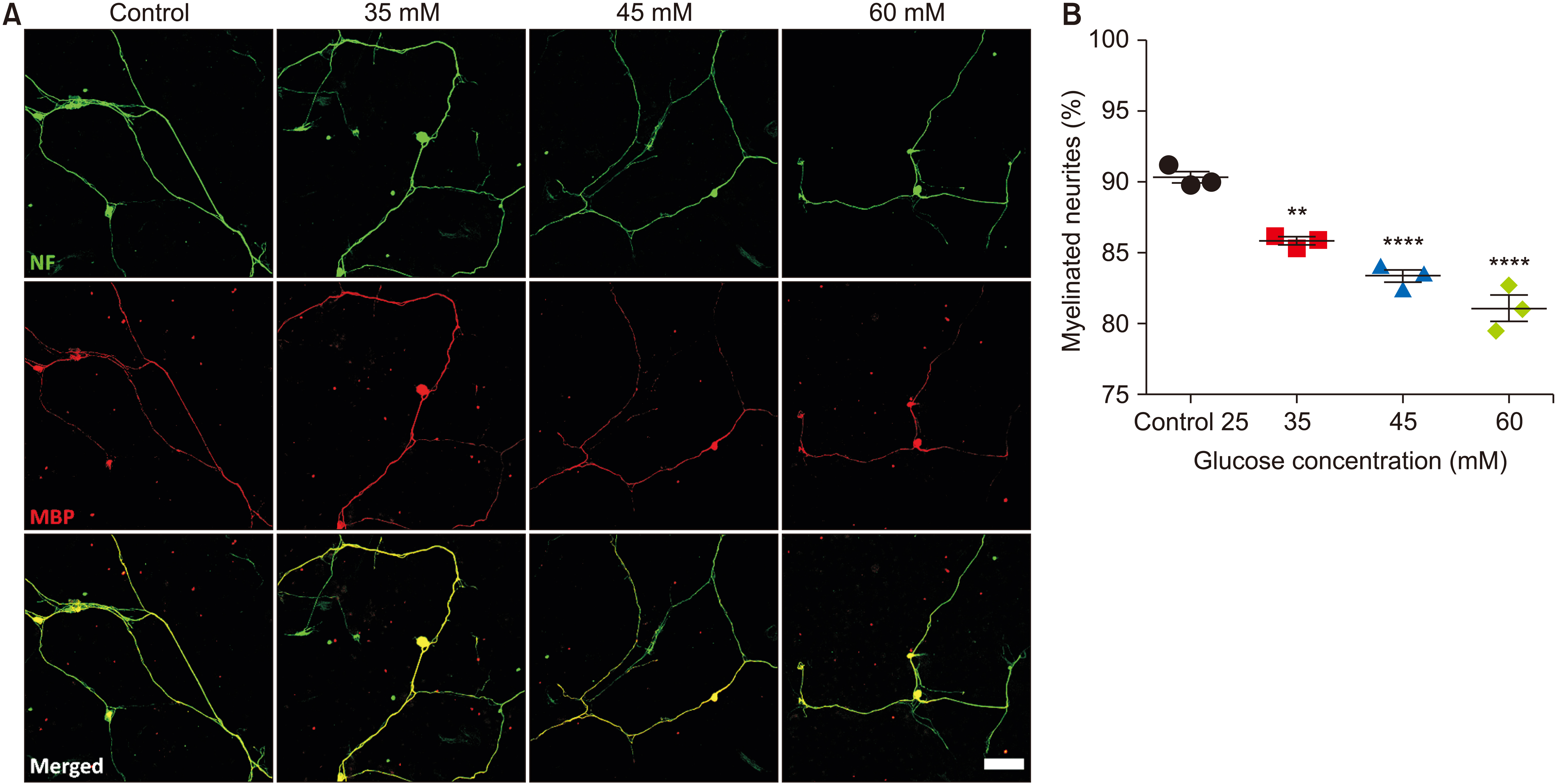
The cells showed similar symptoms observed in in vivo models of this complication. In a single culture, hyperglycemia attenuated the proliferation and maturation of SCs, induced apoptosis, and impaired neurite extension of both sensory and motor neurons. In a co-culture of SCs and neurons, the percentage of myelinated neurites in the hyperglycemia-treated group was significantly lower than that in the control group. This impaired neurite extension and myelination was reversed by the introduction of insulin to the hyperglycemic culture media.
Conclusions
Insulin may be a potential candidate for improving diabetic neuropathy. Insulin can function as a neurotrophic factor to support both neurons and SCs. Further research is needed to discover the potential of insulin in improving diabetic neuropathy.
Keyword
Figure
Reference
-
1. Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM. 2015; Diabetic neuropathic pain: physiopathology and treatment. World J Diabetes. 6:432–44. DOI: 10.4239/wjd.v6.i3.432. PMID: 25897354. PMCID: PMC4398900.
Article2. Hicks CW, Selvin E. 2019; Epidemiology of peripheral neuropathy and lower extremity disease in diabetes. Curr Diab Rep. 19:86. DOI: 10.1007/s11892-019-1212-8. PMID: 31456118. PMCID: PMC6755905.
Article3. Feldman EL, Nave KA, Jensen TS, Bennett DLH. 2017; New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 93:1296–313. DOI: 10.1016/j.neuron.2017.02.005. PMID: 28334605. PMCID: PMC5400015.
Article4. Zychowska M, Rojewska E, Przewlocka B, Mika J. 2013; Mechanisms and pharmacology of diabetic neuropathy - experimental and clinical studies. Pharmacol Rep. 65:1601–10. DOI: 10.1016/S1734-1140(13)71521-4. PMID: 24553008.
Article5. Gaesser JM, Fyffe-Maricich SL. 2016; Intracellular signaling pathway regulation of myelination and remyelination in the CNS. Exp Neurol. 283(Pt B):501–11. DOI: 10.1016/j.expneurol.2016.03.008. PMID: 26957369. PMCID: PMC5010983.
Article6. Geuna S, Raimondo S, Fregnan F, Haastert-Talini K, Grothe C. 2016; In vitro models for peripheral nerve regeneration. Eur J Neurosci. 43:287–96. DOI: 10.1111/ejn.13054. PMID: 26309051.7. Sugimoto K, Murakawa Y, Sima AA. 2002; Expression and localization of insulin receptor in rat dorsal root ganglion and spinal cord. J Peripher Nerv Syst. 7:44–53. DOI: 10.1046/j.1529-8027.2002.02005.x. PMID: 11939351.
Article8. Pham VM, Matsumura S, Katano T, Funatsu N, Ito S. 2019; Diabetic neuropathy research: from mouse models to targets for treatment. Neural Regen Res. 14:1870–9. DOI: 10.4103/1673-5374.259603. PMID: 31290436. PMCID: PMC6676867.
Article9. Hao W, Tashiro S, Hasegawa T, Sato Y, Kobayashi T, Tando T, et al. 2015; Hyperglycemia promotes Schwann cell de-differentiation and de-myelination via sorbitol accumulation and Igf1 protein down-regulation. J Biol Chem. 290:17106–15. DOI: 10.1074/jbc.M114.631291. PMID: 25998127. PMCID: PMC4498049.
Article10. Almhanna K, Wilkins PL, Bavis JR, Harwalkar S, Berti-Mattera LN. 2002; Hyperglycemia triggers abnormal signaling and proliferative responses in Schwann cells. Neurochem Res. 27:1341–7. DOI: 10.1023/A:1021671615939. PMID: 12512939.11. Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A, et al. 2002; High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 16:1738–48. DOI: 10.1096/fj.01-1027com. PMID: 12409316.
Article12. Shetter AR, Muttagi G, Sagar CB. 2011; Expression and localization of insulin receptors in dissociated primary cultures of rat Schwann cells. Cell Biol Int. 35:299–304. DOI: 10.1042/CBI20100523. PMID: 20977434.
Article13. Vincent AM, Brownlee M, Russell JW. 2002; Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci. 959:368–83. DOI: 10.1111/j.1749-6632.2002.tb02108.x. PMID: 11976211.
Article14. Kamiya H, Nakamura J, Hamada Y, Nakashima E, Naruse K, Kato K, et al. 2003; Polyol pathway and protein kinase C activity of rat Schwannoma cells. Diabetes Metab Res Rev. 19:131–9. DOI: 10.1002/dmrr.354. PMID: 12673781.
Article15. Sango K, Suzuki T, Yanagisawa H, Takaku S, Hirooka H, Tamura M, et al. 2006; High glucose-induced activation of the polyol pathway and changes of gene expression profiles in immortalized adult mouse Schwann cells IMS32. J Neurochem. 98:446–58. DOI: 10.1111/j.1471-4159.2006.03885.x. PMID: 16805838.
Article16. Russell JW, Cheng HL, Golovoy D. 2000; Insulin-like growth factor-I promotes myelination of peripheral sensory axons. J Neuropathol Exp Neurol. 59:575–84. DOI: 10.1093/jnen/59.7.575. PMID: 10901228.
Article17. Shindo H, Thomas TP, Larkin DD, Karihaloo AK, Inada H, Onaya T, et al. 1996; Modulation of basal nitric oxide-dependent cyclic-GMP production by ambient glucose, myo-inositol, and protein kinase C in SH-SY5Y human neuroblastoma cells. J Clin Invest. 97:736–45. DOI: 10.1172/JCI118472. PMID: 8609230. PMCID: PMC507111.
Article18. Koshimura K, Tanaka J, Murakami Y, Kato Y. 2003; Effect of high concentration of glucose on dopamine release from pheochromocytoma-12 cells. Metabolism. 52:922–6. DOI: 10.1016/S0026-0495(03)00059-3. PMID: 12870171.
Article19. Koshimura K, Tanaka J, Murakami Y, Kato Y. 2002; Involvement of nitric oxide in glucose toxicity on differentiated PC12 cells: prevention of glucose toxicity by tetrahydrobiopterin, a cofactor for nitric oxide synthase. Neurosci Res. 43:31–8. DOI: 10.1016/S0168-0102(02)00016-0. PMID: 12074839.
Article20. Röder PV, Wu B, Liu Y, Han W. 2016; Pancreatic regulation of glucose homeostasis. Exp Mol Med. 48:e219. DOI: 10.1038/emm.2016.6. PMID: 26964835. PMCID: PMC4892884.
Article21. Grote CW, Wright DE. 2016; A role for insulin in diabetic neuropathy. Front Neurosci. 10:581. DOI: 10.3389/fnins.2016.00581. PMID: 28066166. PMCID: PMC5179551.
Article22. McCloy RA, Rogers S, Caldon CE, Lorca T, Castro A, Burgess A. 2014; Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle. 13:1400–12. DOI: 10.4161/cc.28401. PMID: 24626186. PMCID: PMC4050138.
Article23. More HL, Chen J, Gibson E, Donelan JM, Beg MF. 2011; A semi-automated method for identifying and measuring myelinated nerve fibers in scanning electron microscope images. J Neurosci Methods. 201:149–58. DOI: 10.1016/j.jneumeth.2011.07.026. PMID: 21839777.
Article24. Bhatheja K, Field J. 2006; Schwann cells: origins and role in axonal maintenance and regeneration. Int J Biochem Cell Biol. 38:1995–9. DOI: 10.1016/j.biocel.2006.05.007. PMID: 16807057.
Article25. Tian L, Prabhakaran MP, Ramakrishna S. 2015; Strategies for regeneration of components of nervous system: scaffolds, cells and biomolecules. Regen Biomater. 2:31–45. DOI: 10.1093/rb/rbu017. PMID: 26813399. PMCID: PMC4669026.
Article26. Duraikannu A, Krishnan A, Chandrasekhar A, Zochodne DW. 2019; Beyond trophic factors: exploiting the intrinsic regenerative properties of adult neurons. Front Cell Neurosci. 13:128. DOI: 10.3389/fncel.2019.00128. PMID: 31024258. PMCID: PMC6460947.
Article27. Nave KA. 2010; Myelination and the trophic support of long axons. Nat Rev Neurosci. 11:275–83. DOI: 10.1038/nrn2797. PMID: 20216548.
Article28. Veves A, King GL. 2001; Can VEGF reverse diabetic neuropathy in human subjects? J Clin Invest. 107:1215–8. DOI: 10.1172/JCI13038. PMID: 11375408. PMCID: PMC209306.
Article29. Chklovskii DB. 2004; Synaptic connectivity and neuronal morphology: two sides of the same coin. Neuron. 43:609–17. DOI: 10.1016/S0896-6273(04)00498-2. PMID: 15339643.
Article30. Kastellakis G, Cai DJ, Mednick SC, Silva AJ, Poirazi P. 2015; Synaptic clustering within dendrites: an emerging theory of memory formation. Prog Neurobiol. 126:19–35. DOI: 10.1016/j.pneurobio.2014.12.002. PMID: 25576663. PMCID: PMC4361279.
Article31. Pham VM, Tu NH, Katano T, Matsumura S, Saito A, Yamada A, et al. 2018; Impaired peripheral nerve regeneration in type-2 diabetic mouse model. Eur J Neurosci. 47:126–39. DOI: 10.1111/ejn.13771. PMID: 29119607.
Article32. Serafín A, Molín J, Márquez M, Blasco E, Vidal E, Foradada L, et al. 2010; Diabetic neuropathy: electrophysiological and morphological study of peripheral nerve degeneration and regeneration in transgenic mice that express IFNbeta in beta cells. Muscle Nerve. 41:630–41. DOI: 10.1002/mus.21564. PMID: 19918773.
Article33. Muthuraman A, Ramesh M, Sood S. 2010; Development of animal model for vasculatic neuropathy: induction by ischemic-reperfusion in the rat femoral artery. J Neurosci Methods. 186:215–21. DOI: 10.1016/j.jneumeth.2009.12.004. PMID: 20026113.
Article34. Salzer JL, Zalc B. 2016; Myelination. Curr Biol. 26:R971–5. DOI: 10.1016/j.cub.2016.07.074. PMID: 27780071. PMCID: PMC8445327.
Article35. Namgung U. 2014; The role of Schwann cell-axon interaction in peripheral nerve regeneration. Cells Tissues Organs. 200:6–12. DOI: 10.1159/000370324. PMID: 25765065.
Article36. Hyung S, Yoon Lee B, Park JC, Kim J, Hur EM, Francis Suh JK. 2015; Coculture of primary motor neurons and Schwann cells as a model for in vitro myelination. Sci Rep. 5:15122. DOI: 10.1038/srep15122. PMID: 26456300. PMCID: PMC4601011.
Article37. Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, et al. 2007; Mouse models of diabetic neuropathy. Neurobiol Dis. 28:276–85. DOI: 10.1016/j.nbd.2007.07.022. PMID: 17804249. PMCID: PMC3730836.
Article38. O'Brien PD, Sakowski SA, Feldman EL. 2014; Mouse models of diabetic neuropathy. ILAR J. 54:259–72. DOI: 10.1093/ilar/ilt052. PMID: 24615439. PMCID: PMC3962259.39. Schmidt RE, Green KG, Snipes LL, Feng D. 2009; Neuritic dystrophy and neuronopathy in Akita (Ins2(Akita)) diabetic mouse sympathetic ganglia. Exp Neurol. 216:207–18. DOI: 10.1016/j.expneurol.2008.11.019. PMID: 19111542. PMCID: PMC2672346.
Article40. Murakami T, Iwanaga T, Ogawa Y, Fujita Y, Sato E, Yoshitomi H, et al. 2013; Development of sensory neuropathy in streptozotocin-induced diabetic mice. Brain Behav. 3:35–41. DOI: 10.1002/brb3.111. PMID: 23407314. PMCID: PMC3568788.
Article41. De Gregorio C, Contador D, Campero M, Ezquer M, Ezquer F. 2018; Characterization of diabetic neuropathy progression in a mouse model of type 2 diabetes mellitus. Biol Open. 7:bio036830. DOI: 10.1242/bio.036830. PMID: 30082375. PMCID: PMC6176942.
Article42. Tong Z, Segura-Feliu M, Seira O, Homs-Corbera A, Del Río JA, Samitier J. 2015; A microfluidic neuronal platform for neuron axotomy and controlled regenerative studies. RSC Adv. 5:73457–66. https://pubs.rsc.org/en/content/articlehtml/2015/ra/c5ra11522a. DOI: 10.1039/C5RA11522A.
Article43. inivasan S Sr, Stevens M, Wiley JW. 2000; Diabetic peripheral neuropathy: evidence for apoptosis and associated mitochondrial dysfunction. Diabetes. 49:1932–8. DOI: 10.2337/diabetes.49.11.1932. PMID: 11078462.
Article44. Zochodne DW. 2014; Mechanisms of diabetic neuron damage: molecular pathways. Handb Clin Neurol. 126:379–99. DOI: 10.1016/B978-0-444-53480-4.00028-X. PMID: 25410235.45. Ramji N, Toth C, Kennedy J, Zochodne DW. 2007; Does diabetes mellitus target motor neurons? Neurobiol Dis. 26:301–11. DOI: 10.1016/j.nbd.2006.11.016. PMID: 17337195.
Article46. Hameed S. 2019; Nav1.7 and Nav1.8: role in the pathophysiology of pain. Mol Pain. 15:1744806919858801. DOI: 10.1177/1744806919858801. PMID: 31172839. PMCID: PMC6589956.47. Zochodne DW. 2015; Diabetes and the plasticity of sensory neurons. Neurosci Lett. 596:60–5. DOI: 10.1016/j.neulet.2014.11.017. PMID: 25445357.
Article48. Kerner W, Brückel J. German Diabetes Association. 2014; Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 122:384–6. DOI: 10.1055/s-0034-1366278. PMID: 25014088.
Article49. Krishnamurthy M, Li J, Al-Masri M, Wang R. 2008; Expression and function of alphabeta1 integrins in pancretic beta (INS-1) cells. J Cell Commun Signal. 2:67–79. DOI: 10.1007/s12079-008-0030-6. PMID: 19023675. PMCID: PMC2648043.50. Shettar A, Muttagi G. 2012; Developmental regulation of insulin receptor gene in sciatic nerves and role of insulin on glycoprotein P0 in the Schwann cells. Peptides. 36:46–53. DOI: 10.1016/j.peptides.2012.04.012. PMID: 22564491.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Insulin and Testosterone on Damage to the Erection-Related Nervous System in Experimentally Diabetic Rats
- Acute Painful Neuropathy Induced by Rapid Correction of Serum Glucose Levels in a Diabetic Patient
- Relationship between Cardiovascular Autonomic Neuropathy and Diabetic Retinopathy in Patients with Non-Insulin Dependent Diabetes Mellitus
- Diabetic Neuropathy: Classification and Pathogenesis
- Clinical spectrum and diagnosis of diabetic neuropathies