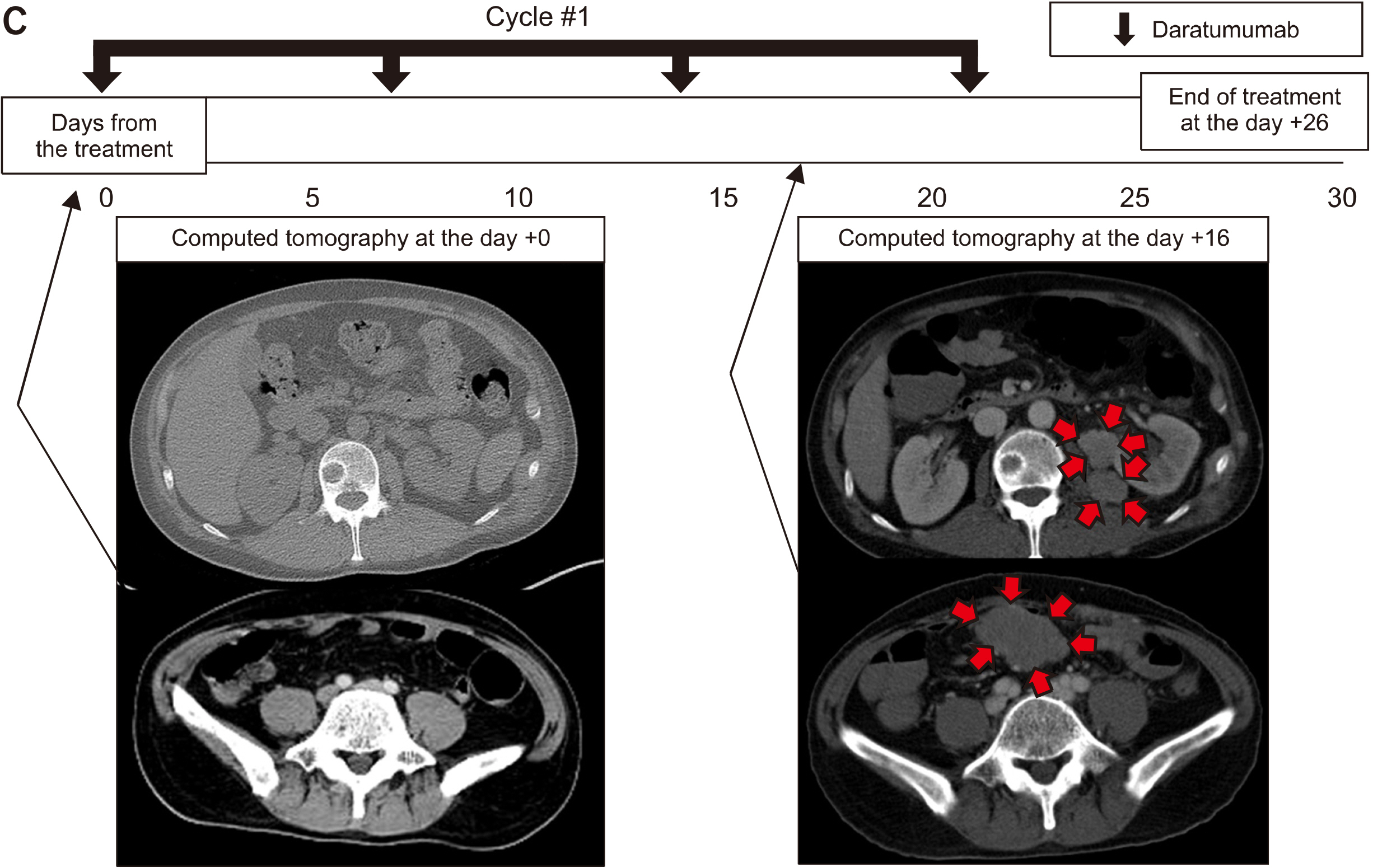
Blood Res.
2022 Mar;57(1):76-80. 10.5045/br.2022.2021183.
Daratumumab monotherapy in relapsed and refractory multiple myeloma patients with severely compromised forced expiratory volume in one second
- Affiliations
-
- 1Department of Hematology, Seoul St. Mary’s Hematology Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- KMID: 2527501
- DOI: http://doi.org/10.5045/br.2022.2021183
Figure
Reference
-
1. McKeage K. 2016; Daratumumab: first global approval. Drugs. 76:275–81. DOI: 10.1007/s40265-015-0536-1. PMID: 26729183.
Article2. Lokhorst HM, Plesner T, Laubach JP, et al. 2015; Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med. 373:1207–19. DOI: 10.1056/NEJMoa1506348. PMID: 26308596.
Article3. Lonial S, Weiss BM, Usmani SZ, et al. 2016; Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet. 387:1551–60. DOI: 10.1016/S0140-6736(15)01120-4. PMID: 26778538.
Article4. Lin P, Owens R, Tricot G, Wilson CS. 2004; Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 121:482–8. DOI: 10.1309/74R4TB90BUWH27JX. PMID: 15080299.
Article5. White TA, Johnson S, Walseth TF, et al. 2000; Subcellular localization of cyclic ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase activities in porcine airway smooth muscle. Biochim Biophys Acta. 1498:64–71. DOI: 10.1016/S0167-4889(00)00077-X. PMID: 11042351.
Article6. Guedes AG, Deshpande DA, Dileepan M, et al. 2015; CD38 and airway hyper-responsiveness: studies on human airway smooth muscle cells and mouse models. Can J Physiol Pharmacol. 93:145–53. DOI: 10.1139/cjpp-2014-0410. PMID: 25594684. PMCID: PMC4648235.
Article7. Durer C, Durer S, Lee S, et al. 2020; Treatment of relapsed multiple myeloma: evidence-based recommendations. Blood Rev. 39:100616. DOI: 10.1016/j.blre.2019.100616. PMID: 31500848.
Article8. Park SS, Byun JM, Yoon SS, et al. 2021; Daratumumab monotherapy for relapsed/refractory multiple myeloma, focussed on clinical trial-unfit patients and subsequent therapy. Br J Haematol. 193:101–12. DOI: 10.1111/bjh.17071. PMID: 33368165.
Article9. 2017. Common terminology criteria for adverse events (CTCAE). Version 5.0. National Cancer Institute;Bethesda, MD:10. Byun JM, Yoon SS, Shin DY, et al. 2016; Real world treatment outcomes of multiple myeloma in Korea. Blood (ASH Annual Meeting Abstracts). 128(Suppl):2367. DOI: 10.1182/blood.V128.22.2367.2367.
Article11. Park SS, Eom HS, Kim JS, et al. 2019; Brief report: clinical experiences after emergency use of daratumumab monotherapy for relapsed or refractory multiple myeloma in real practice. Jpn J Clin Oncol. 49:92–5. DOI: 10.1093/jjco/hyy177. PMID: 30476124.
Article12. Byun JM, Yoon SS, Koh Y, et al. 2019; Daratumumab monotherapy in heavily pretreated Asian patients with relapsed and refractory multiple myeloma: a real-world experience. Anticancer Res. 39:5165–70. DOI: 10.21873/anticanres.13712. PMID: 31519629.
Article13. Kuzume A, Tabata R, Terao T, et al. 2021; Safety and efficacy of daratumumab in patients with multiple myeloma and severe renal failure. Br J Haematol. 193:e33–6. DOI: 10.1111/bjh.17412. PMID: 33748953.
Article14. Nooka AK, Gleason C, Sargeant MO, et al. 2018; Managing infusion reactions to new monoclonal antibodies in multiple myeloma: daratumumab and elotuzumab. J Oncol Pract. 14:414–22. DOI: 10.1200/JOP.18.00143. PMID: 29996069.
Article15. Moore DC, Arnall JR, Thompson DL, et al. 2020; Evaluation of montelukast for the prevention of infusion-related reactions with daratumumab. Clin Lymphoma Myeloma Leuk. 20:e777–81. DOI: 10.1016/j.clml.2020.05.024. PMID: 32660902.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of relapsed and refractory multiple myeloma
- A Case of Panagglutination on Antibody Identification in a Multiple Myeloma Patient Receiving Daratumumab
- Predictive role of absolute lymphocyte count in daratumumab-treated patients with relapsed/ refractory multiple myeloma
- Ventilatory Dynamics in Hypertensive Heart Disease
- Forced Expiratory Volume in One Second and ECG Sign of Cor Pulmonale in Coal Workers' Pneumoconiosis