J Korean Med Sci.
2022 Mar;37(11):e92. 10.3346/jkms.2022.37.e92.
A Multi-Center, Double-Blind Randomized Controlled Phase III Clinical Trial to Evaluate the Antiviral Activity and Safety of DA-2802 (Tenofovir Disoproxil Orotate) and Viread (Tenofovir Disoproxil Fumarate) in Chronic Hepatitis B Patients
- Affiliations
-
- 1Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Division of Gastroenterology, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 3Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Catholic University of Korea College of Medicine, Seoul, Korea
- 5Division of Gastroenterology and Hepatology, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea
- 7Department of Internal Medicine, Kosin University Gospel Hospital, Kosin University College of Medicine, Busan, Korea
- 8Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Korea
- 9Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 10Department of Internal Medicine and Liver Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 11Department of Gastroenterology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 12Department of Internal Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea
- 13Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 14Department of Internal Medicine, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
- 15Department of Internal Medicine, Wonkwang University School of Medicine, Iksan, Korea
- 16Department of Internal Medicine, Ilsan Paik Hospital, Inje University College of Medicine, Ilsan, Korea
- 17Department of Internal Medicine, Inha University Hospital, Inha University School of Medicine, Incheon, Korea
- 18Department of Gastroenterology, Jeonbuk National University Hospital, Jeonju, Korea
- 19Department of Internal Medicine, Jeju National University School of Medicine, Jeju, Korea
- 20Department of Internal Medicine, School of Medicine, Chungnam National University, Daejeon, Korea
- KMID: 2527452
- DOI: http://doi.org/10.3346/jkms.2022.37.e92
Abstract
- Background
Tenofovir disoproxil fumarate (TDF, Viread® ) had been used as a standard treatment option of chronic hepatitis B (CHB). This clinical trial was conducted to evaluate the efficacy and safety of DA-2802 (tenofovir disoproxil orotate) compared to TDF.
Methods
The present study was a double blind randomized controlled trial. Patients with CHB were recruited from 25 hospitals in Korea and given DA-2802 at a dose of 319 mg once daily or Viread® at a dose of 300 mg once daily for 48 weeks from March 2017 to January 2019. Change in hepatitis B virus (HBV) DNA level at week 48 after dosing compared to baseline was the primary efficacy endpoint. Secondary efficacy endpoints were proportions of subjects with undetectable HBV DNA, those with normal alanine aminotransferase (ALT) levels, and those with loss of hepatitis B envelop antigen (HBeAg), those with loss of hepatitis B surface antigen (HBsAg). Adverse events (AEs) were also investigated.
Results
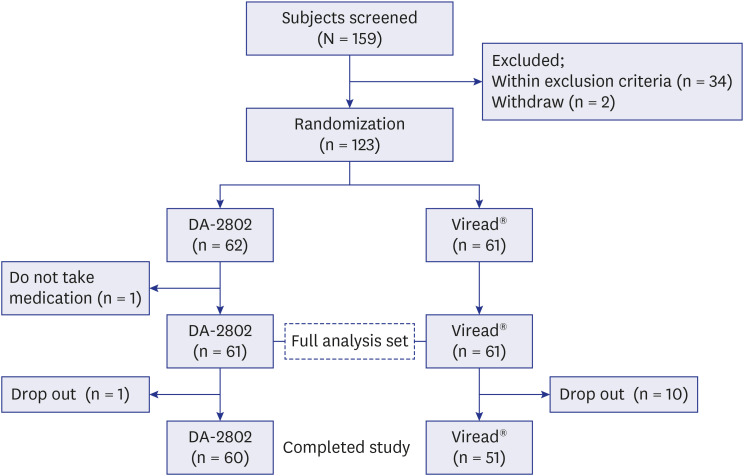
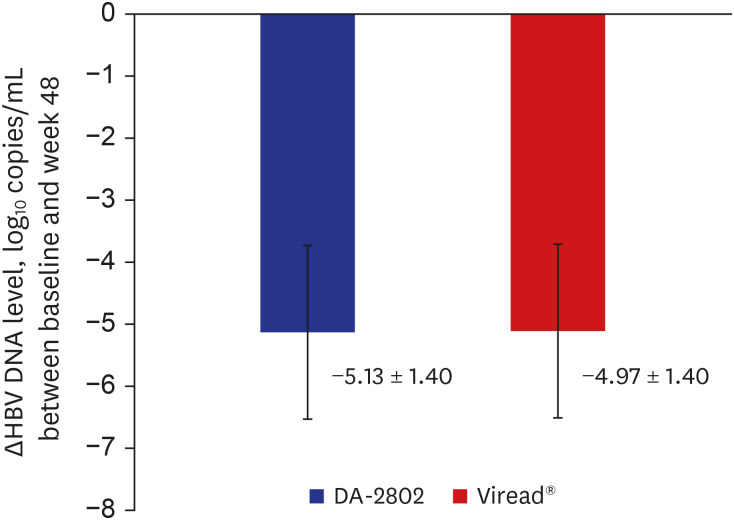
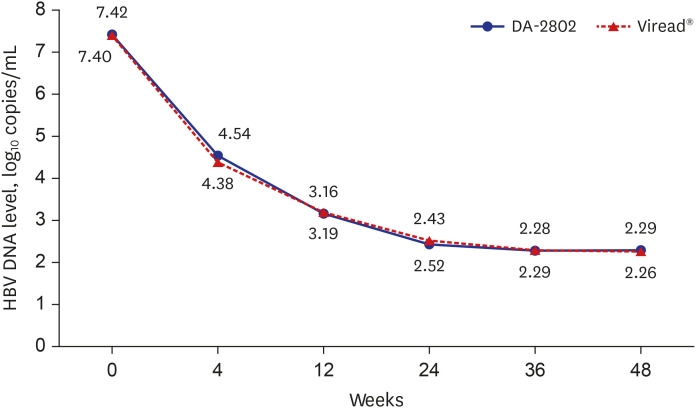
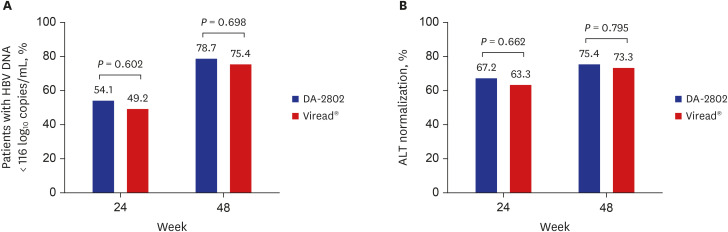
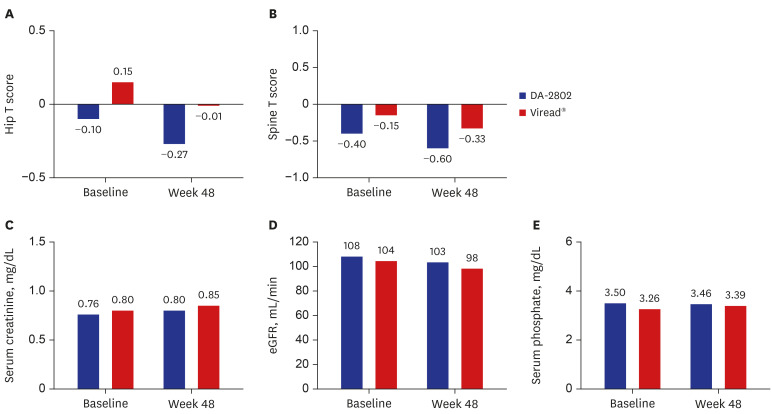
A total of 122 patients (DA-2802 group: n = 61, Viread® group: n = 61) were used as full analysis set for efficacy analysis. Mean age, proportion of males, laboratory results and virologic characteristics were not different between the two groups. The change in HBV DNA level at week 48 from baseline was −5.13 ± 1.40 in the DA-2802 group and −4.97 ± 1.40 log 10 copies/mL in the Viread® group. The analysis of primary endpoint using the nonparametric analysis of covariance showed statistically significant results (P < 0.001), which confirmed non-inferiority of DA-2802 to Viread® by a prespecified noninferiority margin of 1. The proportion of undetectable HBV DNA was 78.7% in the DA-2802 group and 75.4% in the Viread® group (P = 0.698). The proportion of subjects who had normal ALT levels was 75.4% in the DA-2802 group and 73.3% in the Viread® group (P = 0.795). The proportion of those with HBeAg loss was 8.1% in the DA-2802 group and 10.8% in the Viread® group (P = 1.000). No subject showed HBsAg loss. The frequency of AEs during treatment was similar between the two groups. Most AEs were mild to moderate in severity.
Conclusion
DA-2802 is considered an effective and safe treatment for patients with CHB.
Keyword
Figure
Cited by 1 articles
-
Long-Term Real-World Outcomes of Tenofovir Alafenamide in Chronic Hepatitis B: Detailed Analysis of Treatment-Naive and Experienced Patients
Yu-Xuan Song, Guang-Jun Song, Hui Ma, Bo Feng, Yan-Di Xie
Korean J Gastroenterol. 2025;85(1):64-72. doi: 10.4166/kjg.2024.140.
Reference
-
1. Blumberg BS, Alter HJ, Visnich S. “New” antigen in leukemia sera. JAMA. 1965; 191(7):541–546. PMID: 14239025.2. Lee WM. Hepatitis B virus infection. N Engl J Med. 1997; 337(24):1733–1745. PMID: 9392700.3. Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000--summary of a workshop. Gastroenterology. 2001; 120(7):1828–1853. PMID: 11375963.4. Shin SK, Kim JH, Park H, Kwon OS, Lee HJ, Yeon JE, et al. Improvement of liver function and non-invasive fibrosis markers in hepatitis B virus-associated cirrhosis: 2 years of entecavir treatment. J Gastroenterol Hepatol. 2015; 30(12):1775–1781. PMID: 26095700.5. Shim JH, Lee HC, Kim KM, Lim YS, Chung YH, Lee YS, et al. Efficacy of entecavir in treatment-naïve patients with hepatitis B virus-related decompensated cirrhosis. J Hepatol. 2010; 52(2):176–182. PMID: 20006394.6. Kurokawa M, Hiramatsu N, Oze T, Yakushijin T, Miyazaki M, Hosui A, et al. Long-term effect of lamivudine treatment on the incidence of hepatocellular carcinoma in patients with hepatitis B virus infection. J Gastroenterol. 2012; 47(5):577–585. PMID: 22231575.7. Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013; 58(1):98–107. PMID: 23213040.8. Lim YS, Han S, Heo NY, Shim JH, Lee HC, Suh DJ. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology. 2014; 147(1):152–161. PMID: 24583062.9. European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017; 67(2):370–398. PMID: 28427875.10. Terrault NA, Lok AS, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018; 67(4):1560–1599. PMID: 29405329.11. Choi J, Jo C, Lim YS. Tenofovir versus entecavir on recurrence of hepatitis B virus-related hepatocellular carcinoma after surgical resection. Hepatology. 2021; 73(2):661–673. PMID: 32324905.12. de Man RA, Wolters LM, Nevens F, Chua D, Sherman M, Lai CL, et al. Safety and efficacy of oral entecavir given for 28 days in patients with chronic hepatitis B virus infection. Hepatology. 2001; 34(3):578–582. PMID: 11526545.13. Kim YK, Choi MJ, Oh TY, Yu KS, Lee S. A comparative pharmacokinetic and tolerability analysis of the novel orotic acid salt form of tenofovir disoproxil and the fumaric acid salt form in healthy subjects. Drug Des Devel Ther. 2017; 11:3171–3177.14. Kitrinos KM, Corsa A, Liu Y, Flaherty J, Snow-Lampart A, Marcellin P, et al. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology. 2014; 59(2):434–442. PMID: 23939953.15. Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013; 381(9865):468–475. PMID: 23234725.16. Yim HJ, Kim JH, Park JY, Yoon EL, Park H, Kwon JH, et al. Comparison of clinical practice guidelines for the management of chronic hepatitis B: when to start, when to change, and when to stop. Clin Mol Hepatol. 2020; 26(4):411–429. PMID: 32854458.17. Lee SW, Choi J, Kim SU, Lim YS. Entecavir versus tenofovir in patients with chronic hepatitis B: enemies or partners in the prevention of hepatocellular carcinoma. Clin Mol Hepatol. 2021; 27(3):402–412. PMID: 34157830.18. Siest G, Schiele F, Galteau MM, Panek E, Steinmetz J, Fagnani F, et al. Aspartate aminotransferase and alanine aminotransferase activities in plasma: statistical distributions, individual variations, and reference values. Clin Chem. 1975; 21(8):1077–1087. PMID: 1137913.19. Alberti A, Morsica G, Chemello L, Cavalletto D, Noventa F, Pontisso P, et al. Hepatitis C viraemia and liver disease in symptom-free individuals with anti-HCV. Lancet. 1992; 340(8821):697–698. PMID: 1355801.20. Daniel S, Ben-Menachem T, Vasudevan G, Ma CK, Blumenkehl M. Prospective evaluation of unexplained chronic liver transaminase abnormalities in asymptomatic and symptomatic patients. Am J Gastroenterol. 1999; 94(10):3010–3014. PMID: 10520861.21. Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002; 137(1):1–10. PMID: 12093239.22. Lee JK, Shim JH, Lee HC, Lee SH, Kim KM, Lim YS, et al. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology. 2010; 51(5):1577–1583. PMID: 20162730.23. Sohn W, Jun DW, Kwak MJ, Park Q, Lee KN, Lee HL, et al. Upper limit of normal serum alanine and aspartate aminotransferase levels in Korea. J Gastroenterol Hepatol. 2013; 28(3):522–529. PMID: 22497339.24. Kang HS, Um SH, Seo YS, An H, Lee KG, Hyun JJ, et al. Healthy range for serum ALT and the clinical significance of “unhealthy” normal ALT levels in the Korean population. J Gastroenterol Hepatol. 2011; 26(2):292–299. PMID: 21261719.25. Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008; 6(12):1315–1341. PMID: 18845489.26. Shirvani-Dastgerdi E, Winer BY, Celià-Terrassa T, Kang Y, Tabernero D, Yagmur E, et al. Selection of the highly replicative and partially multidrug resistant rtS78T HBV polymerase mutation during TDF-ETV combination therapy. J Hepatol. 2017; 67(2):246–254. PMID: 28392234.27. Park ES, Lee AR, Kim DH, Lee JH, Yoo JJ, Ahn SH, et al. Identification of a quadruple mutation that confers tenofovir resistance in chronic hepatitis B patients. J Hepatol. 2019; 70(6):1093–1102. PMID: 30794889.28. Buti M, Tsai N, Petersen J, Flisiak R, Gurel S, Krastev Z, et al. Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Dig Dis Sci. 2015; 60(5):1457–1464. PMID: 25532501.29. Petersen J, Heyne R, Mauss S, Schlaak J, Schiffelholz W, Eisenbach C, et al. Effectiveness and safety of tenofovir disoproxil fumarate in chronic hepatitis B: a 3-year prospective field practice study in Germany. Dig Dis Sci. 2016; 61(10):3061–3071. PMID: 26576555.30. Lampertico P, Chan HL, Janssen HL, Strasser SI, Schindler R, Berg T. Review article: long-term safety of nucleoside and nucleotide analogues in HBV-monoinfected patients. Aliment Pharmacol Ther. 2016; 44(1):16–34. PMID: 27198929.31. Gill US, Zissimopoulos A, Al-Shamma S, Burke K, McPhail MJ, Barr DA, et al. Assessment of bone mineral density in tenofovir-treated patients with chronic hepatitis B: can the fracture risk assessment tool identify those at greatest risk? J Infect Dis. 2015; 211(3):374–382. PMID: 25156561.32. Verhelst D, Monge M, Meynard JL, Fouqueray B, Mougenot B, Girard PM, et al. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am J Kidney Dis. 2002; 40(6):1331–1333. PMID: 12460055.33. Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. 2008; 22(2):99–103. PMID: 18260800.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Letter: Cardiovascular risk of tenofovir disoproxil fumarate or tenofovir alafenamide fumarate in patients with chronic hepatitis B: More questions than an answer – author’s reply
- Comparative pharmacokinetics between tenofovir disoproxil phosphate and tenofovir disoproxil fumarate in healthy subjects
- Dyslipidemia in chronic hepatitis B patients on tenofovir alafenamide: Facts and puzzles
- Reply to correspondence on “Cardiovascular risk in chronic hepatitis B patients treated with tenofovir disoproxil fumarate or tenofovir alafenamide”
- Can We Trust Safety of Tenofovir Disoproxil in Patients with Decompensated Cirrhosis?