Clin Exp Otorhinolaryngol.
2022 Feb;15(1):60-68. 10.21053/ceo.2020.02313.
Feasibility of Personal Sound Amplification Products in Patients With Moderate Hearing Loss: A Pilot Study
- Affiliations
-
- 1Hearing Research Laboratory, Samsung Medical Center, Seoul, Korea
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2527113
- DOI: http://doi.org/10.21053/ceo.2020.02313
Abstract
Objectives
. This study was conducted to investigate the electroacoustic characteristics of personal sound amplification products (PSAPs), to identify whether PSAPs provide adequate gain and output for three common hearing loss (HL) configurations, and to compare the benefits of a representative PSAP (RPSAP) and a conventional hearing aid (HA) for clinical hearing outcomes as a pilot study.
Methods
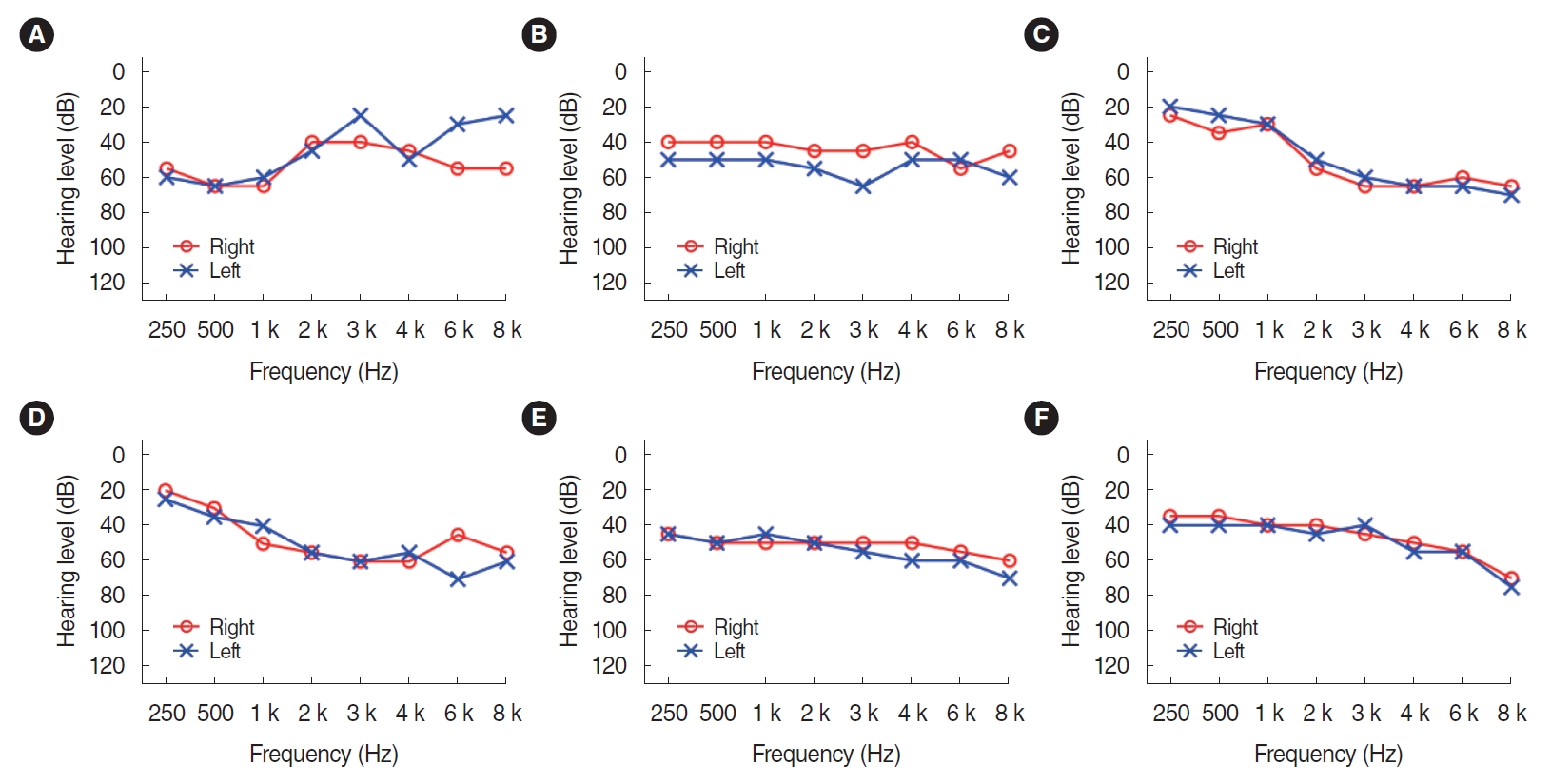
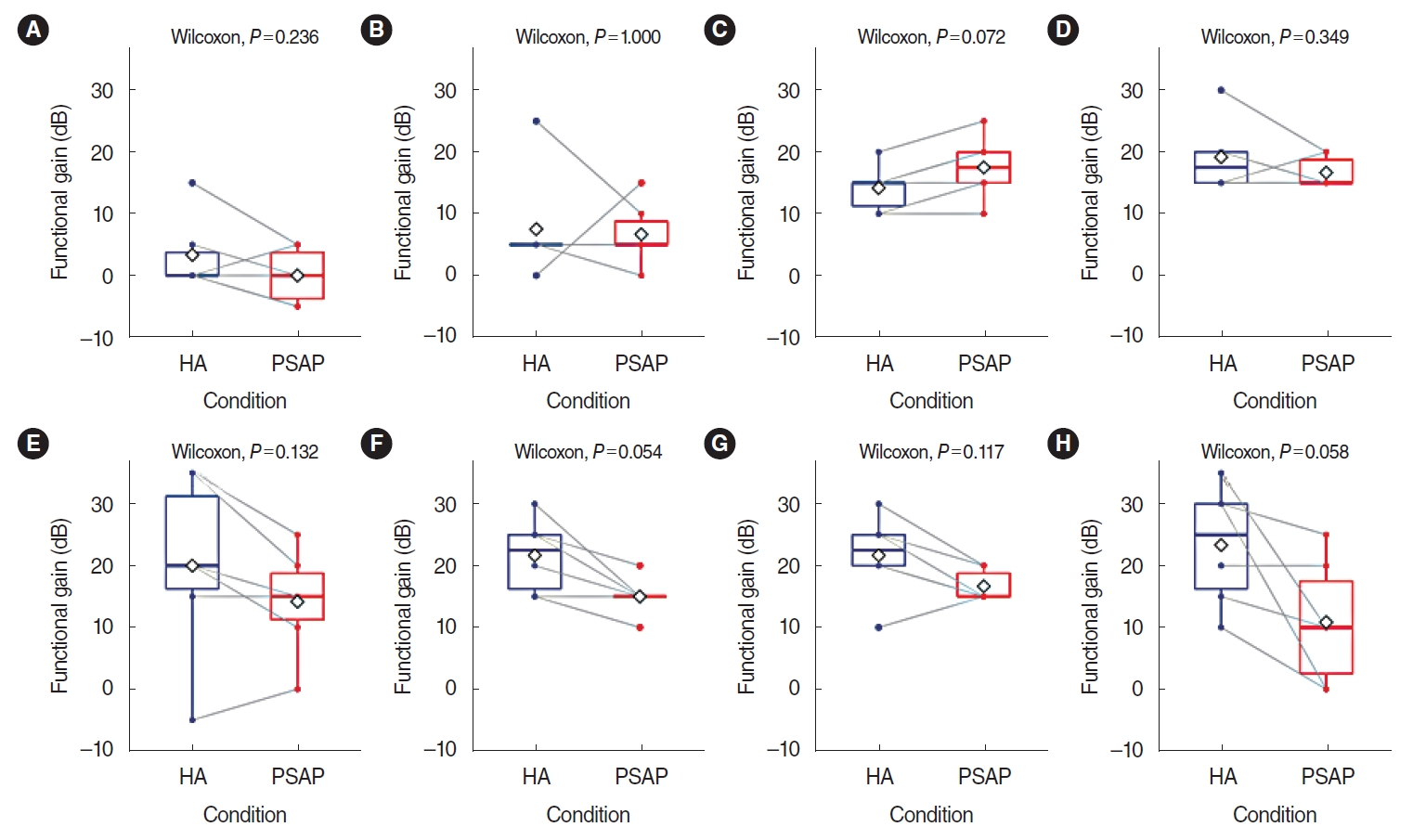
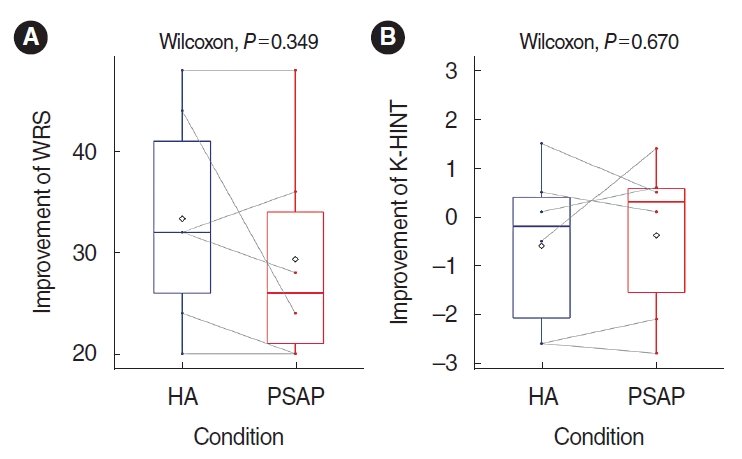
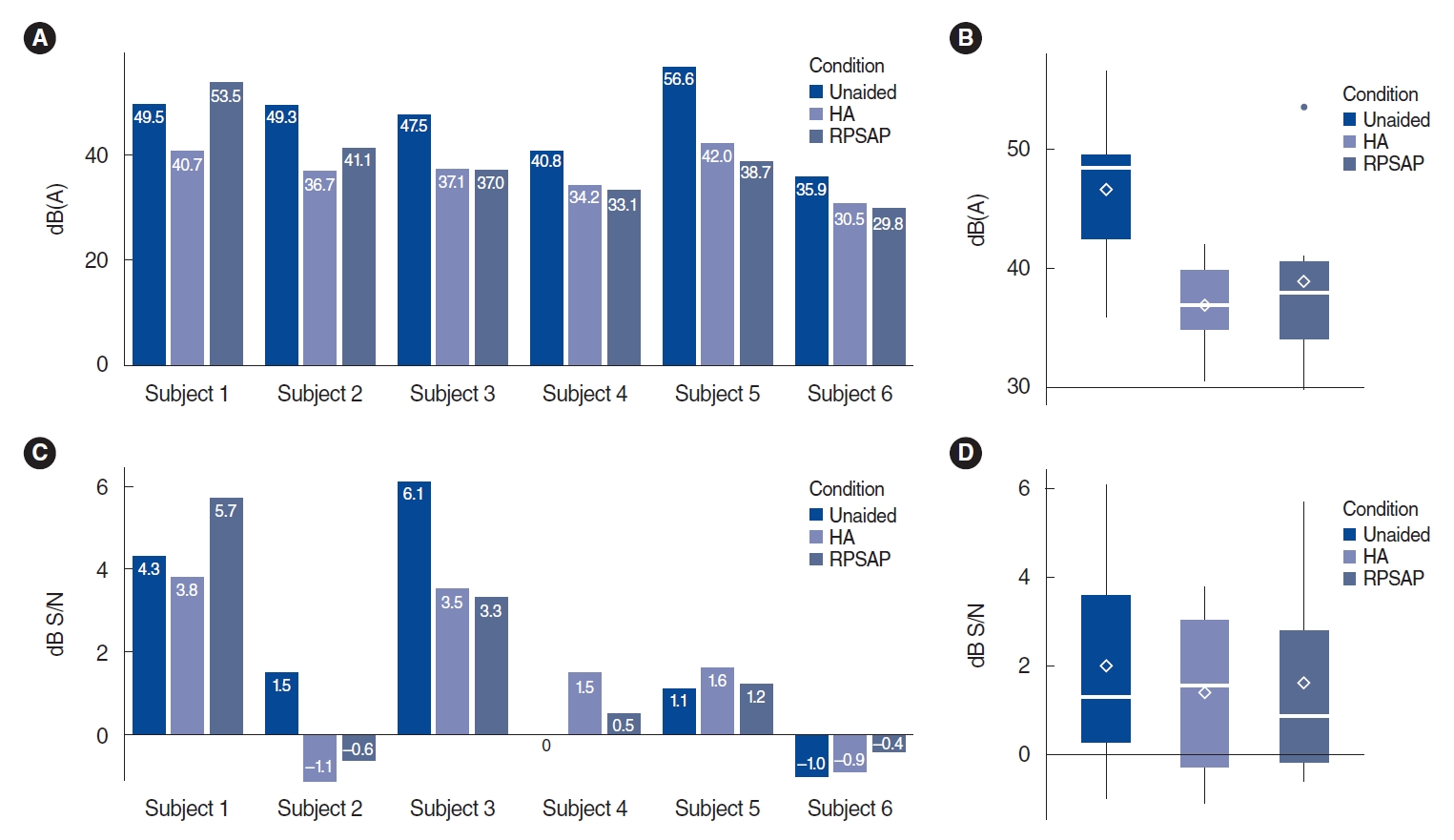
. The study comprised three phases: electroacoustic analysis, simulated real-ear measurements (REMs), and clinical hearing experiments. Electroacoustic analysis and simulated REMs were performed for three basic PSAPs (BeethoSOL, EarJJang, and Geniesori2) and three high-end PSAPs (Hearing Able, Olive Smart Ear, and SoriIn) using the Aurical Hearing Instrument Test box with a 2-mL coupler. Four electroacoustic characteristics (maximum output sound pressure level at 90 dB SPL, frequency range, equivalent input noise, and total harmonic distortion) were investigated. By simulated REMs, appropriate levels of the six PSAPs for three common HL configurations (mild-to-moderate high-frequency HL, moderate to moderately severe sloping HL, and moderate flat HL) were determined. Clinical experiments compared the performance of RPSAP to HA, both of which were fitted by audiologists using REMs. Clinical experiments were administered using functional gain, a word recognition test, and the Korean version of the Hearing in Noise Test in six participants with bilateral moderate sensorineural HL.
Results
. The two high-end devices met all tolerances. One basic and two high-end PSAPs showed appropriate levels for three common HL configurations. In the clinical experiments, the RPSAP showed better performance than unaided, but slightly worse than HA under all test conditions.
Conclusion
. Certain PSAPs met all specified tolerances for electroacoustic analysis and approximated prescriptive targets in well-controlled laboratory conditions. The pilot clinical experiments explored the possibility that the RPSAP could serve as a hearing assistive device for patients with moderate HL.
Figure
Cited by 3 articles
-
Factors Influencing Hearing Aid Adoption in Patients With Hearing Loss in Korea
Young Sang Cho, Ga-Young Kim, Jae Hyuk Choi, Sin Sung Baek, Hye Yoon Seol, Jihyun Lim, Jin Gyun Park, Il Joon Moon
J Korean Med Sci. 2021;37(2):e11. doi: 10.3346/jkms.2022.37.e11.The Effectiveness of Personal Sound Amplification Products in Adults With Mild to Moderate Hearing Loss: Is Their Use Inevitable?
Goun Choe, Moo Kyun Park
Clin Exp Otorhinolaryngol. 2022;15(1):1-2. doi: 10.21053/ceo.2021.01081.Hearables as a Gateway to Hearing Health Care
Hye Yoon Seol, Il Joon Moon
Clin Exp Otorhinolaryngol. 2022;15(2):127-134. doi: 10.21053/ceo.2021.01662.
Reference
-
1. World Health Organization. Deafness and hearing loss. Geneva: World Health Organization;2020 [cited 2020 Apr 3]. Available from: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss.2. Loughrey DG, Kelly ME, Kelley GA, Brennan S, Lawlor BA. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg. 2018; Feb. 144(2):115–26.
Article3. Moon IJ, Baek SY, Cho YS. Hearing aid use and associated factors in South Korea. Medicine (Baltimore). 2015; Oct. 94(42):e1580.
Article4. National Evidence-based Healthcare Collaborating Agency (NECA). Analysis of hearing aid benefit and barriers in hearing aid acquisition in Korea (NECA 2010-008). Seoul: NECA;2010 [cited 2020 Apr 3]. Available from: http://gzone.kr/gzone/gZoneSearchDetailList.do?contentsId=PLC20170213095.5. President’s Council of Advisors on Science and Technology. Aging America & hearing loss: imperative of improved hearing technologies. Washington (DC): President’s Council of Advisors on Science and Technology;2015 [cited 2020 Apr 3]. Available from: https://obamawhitehouse.archives.gov/sites/default/files/microsites/ostp/PCAST/PCAST%20hearing%20letter%20report.pdf.6. Lin FR. Time for a top-down approach to hearing aid affordability and accessibility. Am J Public Health. 2018; Feb. 108(2):166–8.
Article7. Brody L, Wu YH, Stangl E. A comparison of personal sound amplification products and hearing aids in ecologically relevant test environments. Am J Audiol. 2018; Dec. 27(4):581–93.
Article8. Cho YS, Park SY, Seol HY, Lim JH, Cho YS, Hong SH, et al. Clinical performance evaluation of a personal sound amplification product vs a basic hearing aid and a premium hearing aid. JAMA Otolaryngol Head Neck Surg. 2019; Jun. 145(6):516–22.
Article9. Choi JE, Kim J, Yoon SH, Hong SH, Moon IJ. A personal sound amplification product compared to a basic hearing aid for speech intelligibility in adults with mild-to-moderate sensorineural hearing loss. J Audiol Otol. 2020; Apr. 24(2):91–8.
Article10. Callaway SL, Punch JL. An electroacoustic analysis of over-the-counter hearing aids. Am J Audiol. 2008; Jun. 17(1):14–24.
Article11. Reed NS, Betz J, Lin FR, Mamo SK. Pilot electroacoustic analyses of a sample of direct-to-consumer amplification products. Otol Neurotol. 2017; Jul. 38(6):804–8.
Article12. Lee JH. Pure-tone audiometry: methods for air and bone conduction. In : . Practical manual of hearing tests. 2nd ed. Seoul: Hakjisa;2017. p. 110.13. American Speech-Language-Hearing Association. Guidelines for manual pure-tone threshold audiometry. Rockville (MD): American Speech-Language-Hearing Association;1977.14. Kim JS, Lim DH, Hong HN, Shin HW, Lee KD, Hong BN, et al. Development of Korean Standard Monosyllabic Word Lists for Adults (KS-MWL-A). Audiology. 2008; Dec. 4(2):126–40.
Article15. Moon SK, Hee Kim S, Ah Mun H, Jung HK, Lee JH, Choung YH, et al. The Korean hearing in noise test. Int J Audiol. 2008; Jun. 47(6):375–6.
Article16. Chan ZY, McPherson B. Over-the-counter hearing aids: a lost decade for change. Biomed Res Int. 2015; 2015:827463.
Article17. Mamo SK, Reed NS, Nieman CL, Oh ES, Lin FR. Personal sound amplifiers for adults with hearing loss. Am J Med. 2016; Mar. 129(3):245–50.
Article18. Cheng CM, McPherson B. Over-the-counter hearing aids: electroacoustic characteristics and possible target client groups. Audiology. 2000; Mar-Apr. 39(2):110–6.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effectiveness of Personal Sound Amplification Products in Adults With Mild to Moderate Hearing Loss: Is Their Use Inevitable?
- Comparative Effectiveness of Personal Sound Amplification Products Versus Hearing Aids for Unilateral Hearing Loss: A Prospective Randomized Crossover Trial
- Hearing and Speech Perception for People With Hearing Loss Using Personal Sound Amplification Products
- Apple AirPods Pro as a Hearing Assistive Device in Patients with Mild to Moderate Hearing Loss
- A Personal Sound Amplification Product Compared to aBasic Hearing Aid for Speech Intelligibility in Adults withMild-to-Moderate Sensorineural Hearing Loss