Lab Med Online.
2021 Jan;11(1):1-10. 10.47429/lmo.2021.11.1.1.
Standards and Guidelines for Reporting Diagnostic Test Results in Acute Leukemia Patients: Bone Marrow Examination, Flow Cytometry, and Cytogenetic/Molecular Genetics Tests
- Affiliations
-
- 1Department of Laboratory Medicine, Soonchunghyang University Seoul Hospital, Seoul, Korea
- 2Department of Laboratory Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea
- 3Department of Laboratory Medicine, University of Ulsan College of Medicine and Ulsan University Hospital, Ulsan, Korea
- 4Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea
- 5Department of Laboratory Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- 6Department of Laboratory Medicine, Ewha Womans University, College of Medicine, Mokdong Hospital, Seoul, Korea
- 7Department of Laboratory Medicine, Dongkuk University Ilsan Hospital, Goyang, Korea
- 8Department of Laboratory Medicine, Wonkwang University Hospital, Iksan, Korea
- 9Department of Laboratory Medicine, Catholic University Seoul St. Mary’s Hospital, Seoul, Korea
- 10Department of Laboratory Medicine, National Cancer Center Hospital, Goyang, Korea
- KMID: 2525774
- DOI: http://doi.org/10.47429/lmo.2021.11.1.1
Abstract
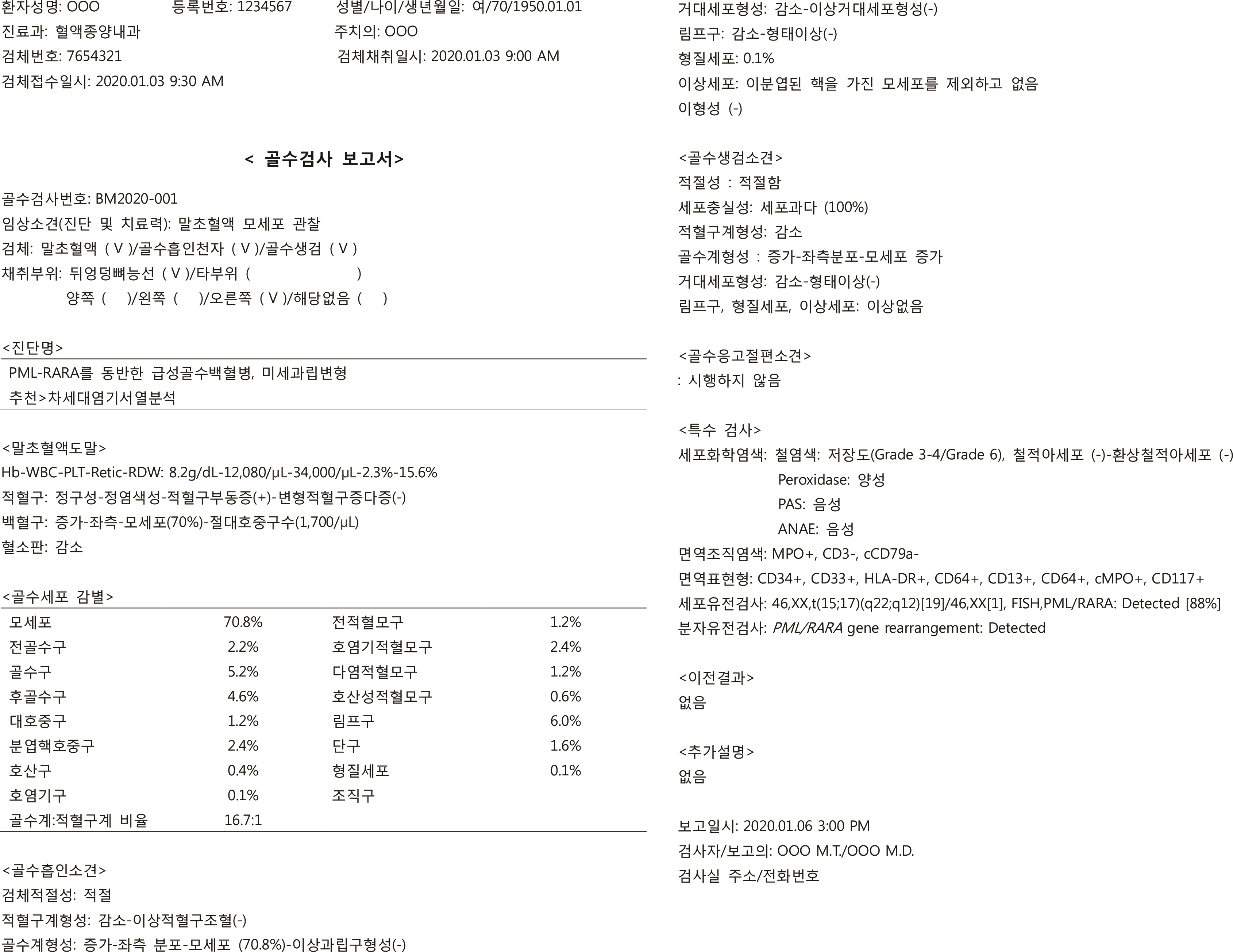
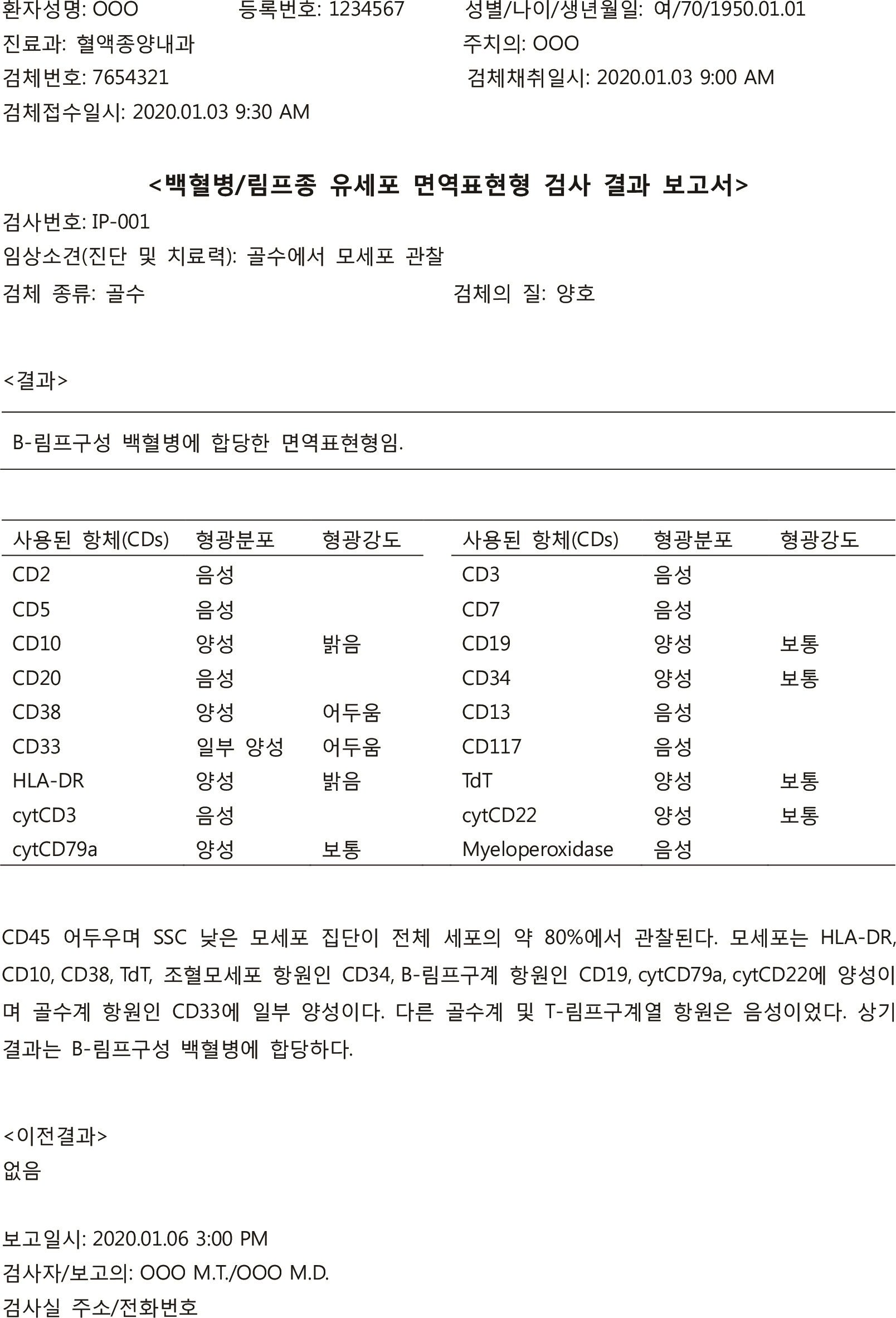
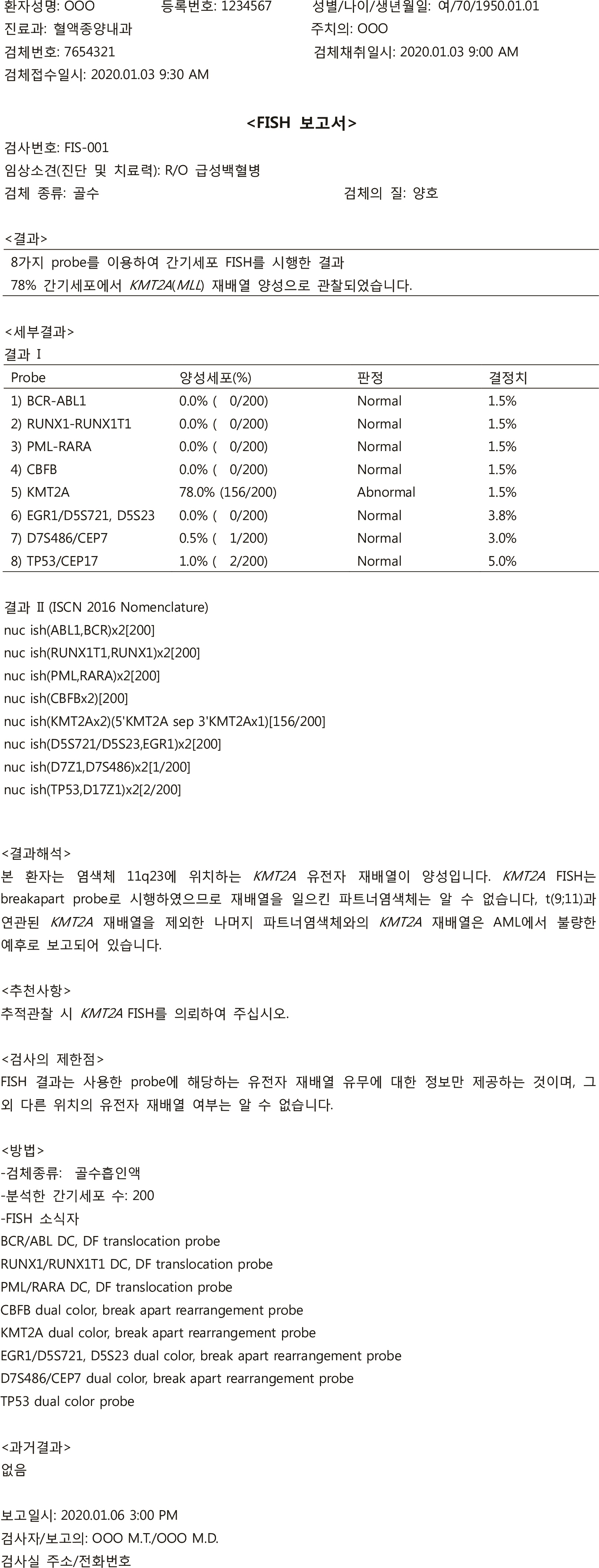
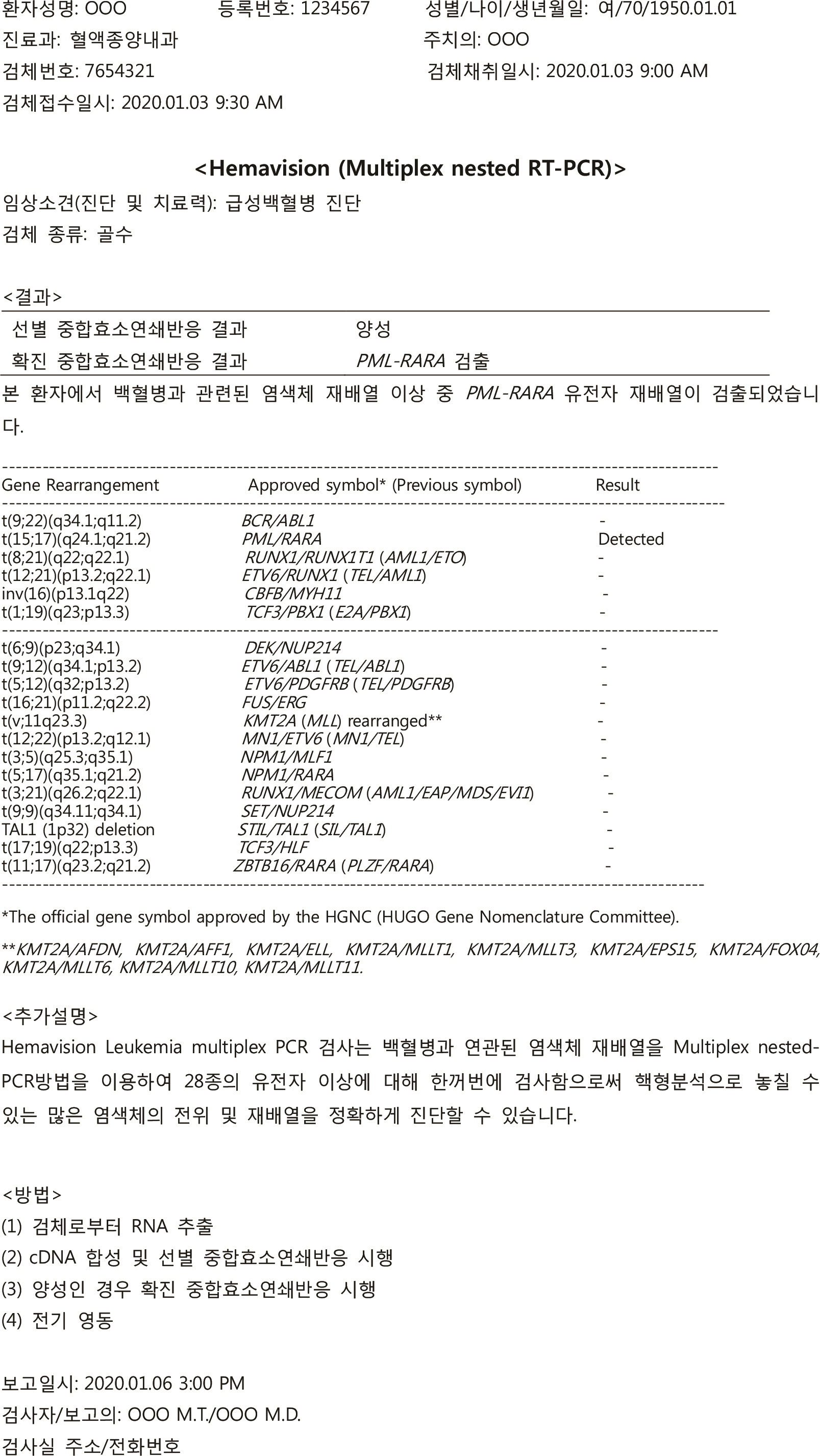
- Reports on hematological neoplasms are produced in various formats in different laboratories. For best patient care, standardization of reports adopting a format that provides concise and clear information is necessary. To this end, the Diagnostic Hematology Standardization Committee, organized by the Korean Society for Laboratory Hematology, has proposed a standardized format for reporting diagnostic test results for acute leukemia patients. It is hoped that this standardization will broadly improve communication with clinicians and improve patient care.
Keyword
Figure
Cited by 1 articles
-
Body Fluid Analysis for Cellular Composition Using Manual Methods: Current Status and Clinical Laboratory Guidelines in Korea (2021)
Hae In Bang, Hyun-Young Kim, Saeam Shin, Ja Young Lee, In-Suk Kim, Young-Uk Cho, Ji Myung Kim, Myung-Geun Shin, Jeong Nyeo Lee, Sang Mee Hwang, Sun-Young Kong
Lab Med Online. 2022;12(4):262-268. doi: 10.47429/lmo.2022.12.4.262.
Reference
-
1. Swerdlow SH, Campo E, editors. 2017. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. International Agency for Research on Cancer;Lyon, France:2. Sever C, Abbott CL, de Baca ME, Khoury JD, Perkins SL, Reichard KK, et al. 2016; Bone marrow synoptic reporting for hematologic neoplasms: Guideline from the College of American Pathologists Pathology and Laboratory Quality Center. Arch Pathol Lab Med. 140:932–49. DOI: 10.5858/arpa.2015-0450-SA. PMID: 26905483.3. Lee SH, Erber WN, Porwit A, Tomonaga M, Peterson LC. 2008; ICSH guidelines for the standardization of bone marrow specimens and reports. Int J Lab Hematol. 30:349–64. DOI: 10.1111/j.1751-553X.2008.01100.x. PMID: 18822060.4. Wood BL, Arroz M, Barnett D, DiGiuseppe J, Greig B, Kussick SJ, et al. 2007; 2006 Bethesda International Consensus recommendations on the immunophenotypic analysis of hematolymphoid neoplasia by flow cytometry: optimal reagents and reporting for the flow cytometric diagnosis of hematopoietic neoplasia. Cytometry B Clin Cytom. 72:S14–22. DOI: 10.1002/cyto.b.20363. PMID: 17803189.5. Clinical and Laboratory Standards Institute. 2007. Clinical flow cytometric analysis of neoplastic hematolymphoid cells;Approved guideline-Second edition. CLSI document H43-A2. Clinical and Laboratory Standards Institute;Wayne, PA:6. Claustres M, Kožich V, Dequeker E, Fowler B, Hehir-Kwa JY, Miller K, et al. 2014; Recommendations for reporting results of diagnostic genetic testing (biochemical, cytogenetic and molecular genetic). Eur J Hum Genet. 22:160–70. DOI: 10.1038/ejhg.2013.125. PMID: 23942201. PMCID: PMC3895644.7. Mikhail FM, Heerema NA, Rao KW, Burnside RD, Cherry AM, Cooley LD. 2016; Section E6.1-6.4 of the ACMG technical standards and guidelines: chromosome studies of neoplastic blood and bone marrow-acquired chromosomal abnormalities. Genet Med. 18:635–42. DOI: 10.1038/gim.2016.50. PMID: 27124785.8. Kwon JA, Kim YG, Park G, Kim JM, Cho YU, Huh J, et al. 2019; Recommendation for the peripheral blood cell morphology report. Lab Med Online. 9:115–25. DOI: 10.3343/lmo.2019.9.3.115.9. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. 2016; The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 127:2391–405. DOI: 10.1182/blood-2016-03-643544. PMID: 27069254.10. Béné M, Nebe T, Bettelheim P, Buldini B, Bumbea H, Kern W, et al. 2011; Immunophenotyping of acute leukemia and lymphoproliferative disorders: a consensus proposal of the European LeukemiaNet Work Package 10. Leukemia. 25:567–74. DOI: 10.1038/leu.2010.312. PMID: 21252983.11. Theunissen P, Mejstrikova E, Sedek L, van der Sluijs-Gelling AJ, Gaipa G, Bartels M, et al. 2017; Standardized flow cytometry for highly sensitive MRD measurements in B-cell acute lymphoblastic leukemia. Blood. 129:347–57. DOI: 10.1182/blood-2016-07-726307. PMID: 27903527. PMCID: PMC5291958.12. Wood BL. 2016; Principles of minimal residual disease detection for hematopoietic neoplasms by flow cytometry. Cytometry B Clin Cytom. 90:47–53. DOI: 10.1002/cyto.b.21239. PMID: 25906832.13. Schuurhuis GJ, Heuser M, Freeman S, Béné M-C, Buccisano F, Cloos J, et al. 2018; Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 131:1275–91. DOI: 10.1182/blood-2017-09-801498. PMID: 29330221. PMCID: PMC5865231.14. Rack KA, van den Berg E, Haferlach C, Beverloo HB, Costa D, Espinet B, et al. 2019; European recommendations and quality assurance for cytogenomic analysis of haematological neoplasms. Leukemia. 33:1851–67. DOI: 10.1038/s41375-019-0378-z. PMID: 30696948. PMCID: PMC6756035.15. McGowan-Jordan J, Simons A, Schmid M. 2016; ISCN: an international system for human cytogenomic nomenclature (2016). Cytogenetic and Genome Research. 149:Basel, Switzerland;1–2.16. Cross NCP, White HE, Evans PAS, Hancock J, Copland M, Milojkovic D, et al. 2018; Consensus on BCR-ABL1 reporting in chronic myeloid leukaemia in the UK. Br J Haematol. 182:777–88. DOI: 10.1111/bjh.15542. PMID: 30125955. PMCID: PMC6175193.17. Ohgami RS, Arber DA. 2013; Challenges in consolidated reporting of hematopoietic neoplasms. Surg Pathol Clin. 6:795–806. DOI: 10.1016/j.path.2013.08.001. PMID: 26839198.18. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. 2015; Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 17:405–24. DOI: 10.1038/gim.2015.30. PMID: 25741868. PMCID: PMC4544753.19. Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, et al. 2017; Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 19:4–23. DOI: 10.1016/j.jmoldx.2016.10.002. PMID: 27993330. PMCID: PMC5707196.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Measurements of treatment response in childhood acute leukemia
- Discrepant Immunophenotypic Characteristics between the Lymph Node and Bone Marrow in Two Mixed-Phenotype Acute Leukemia Patients
- Diagnostic Approach of Acute Leukemia in Children with Bone and Joint Pain
- Acute Megakaryoblastic Leukemia with CD41a-/CD61-/CD42a+ Blasts in an Infant with Down Syndrome
- Relation among the Tests and Comparison of Positivity of Tests for Multi-Drug Resistance in Newly Diagnosed Acute Leukemia