Ann Surg Treat Res.
2022 Feb;102(2):90-99. 10.4174/astr.2022.102.2.90.
Preventive effect of biodegradable stents on biliary stricture and fibrosis after biliary anastomosis in a porcine model
- Affiliations
-
- 1Digestive Disease Center, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
- 2Department of General Surgery, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
- 3Interventional Research Center, M.I.Tech, Co. Ltd., Pyeongtaek, Korea
- 4Department of Pathology, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea
- 5Korea Textile Development Institute, Daegu, Korea
- KMID: 2525446
- DOI: http://doi.org/10.4174/astr.2022.102.2.90
Abstract
- Purpose
The current drain tubes for preventing surgically biliary anastomotic stricture are not naturally and easily removed. If a drain tube using biodegradable material is easily available and the degradation time of the tube is well controlled, surgical anastomotic stricture and fibrosis could be prevented. The aim of this animal study was to evaluate the preventive effect of novel biodegradable stents (BS) on biliary stricture and fibrosis after duct-to-duct (DD) biliary anastomosis.
Methods
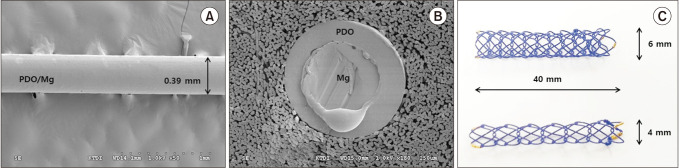
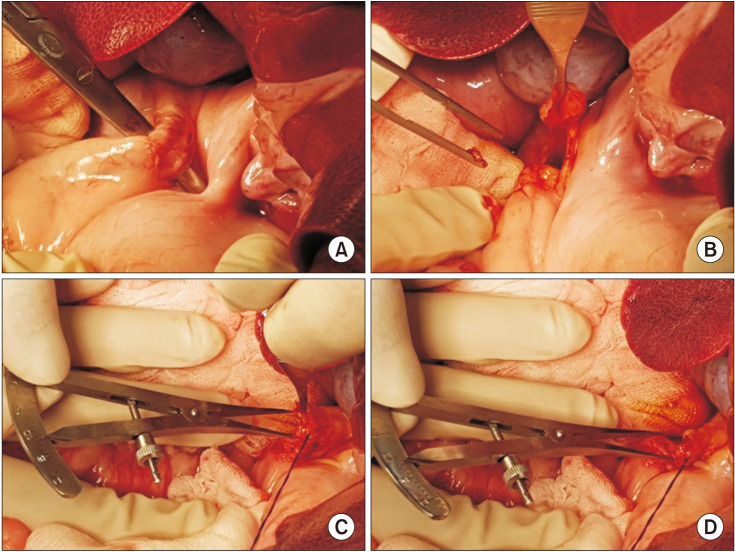
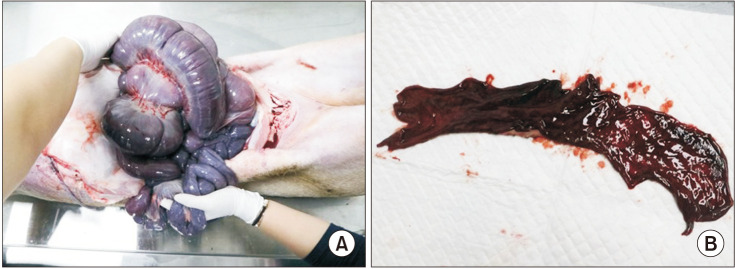
Ten mini-pigs were allocated to the control group (n = 5) and or the stent group (n = 5). The common bile duct was exposed through surgical laparotomy and then resected transversely. In the stent group, a 4-mm or 6-mm polydioxanone/ magnesium sheath-core BS was inserted according to the width of the bile duct, followed by DD biliary anastomosis. In the control group, DD biliary anastomosis was performed without BS insertion.
Results
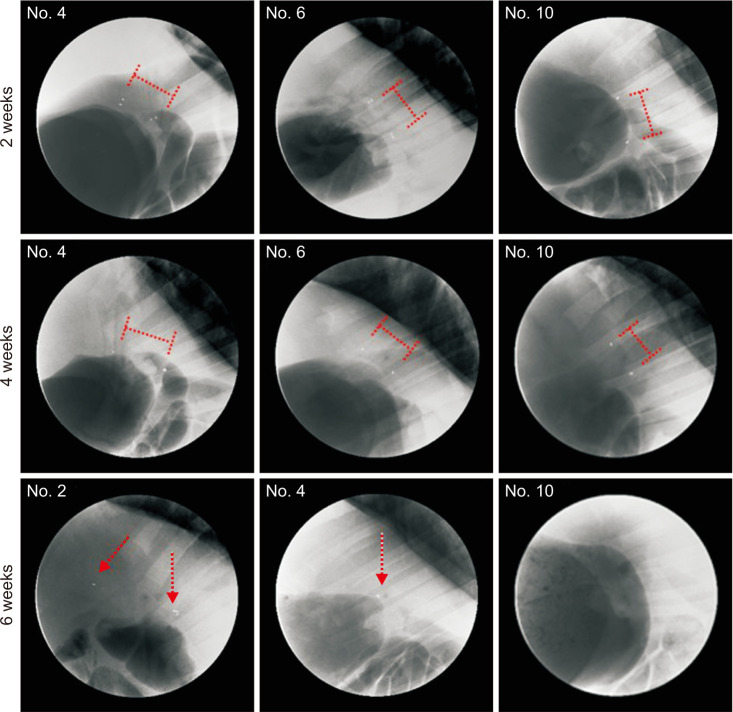
In the stent group, stents were observed without deformity for up to 4 weeks in all animals. Eight weeks later, histopathologic examination revealed that the common bile duct of the anastomosis site was relatively narrower in circumference in the control group compared to the stent group. The degree of fibrosis in the control group was more marked than in the stent group (3.84 mm vs. 0.68 mm, respectively; P < 0.05).
Conclusion
Our study showed that novel BS maintained their original shape and radial force for an adequate time and then disappeared without adverse events. The BS could prevent postoperative complications and strictures after DD biliary anastomosis.
Keyword
Figure
Reference
-
1. Gondolesi GE, Varotti G, Florman SS, Muñoz L, Fishbein TM, Emre SH, et al. Biliary complications in 96 consecutive right lobe living donor transplant recipients. Transplantation. 2004; 77:1842–1848. PMID: 15223901.
Article2. Dulundu E, Sugawara Y, Sano K, Kishi Y, Akamatsu N, Kaneko J, et al. Duct-to-duct biliary reconstruction in adult living-donor liver transplantation. Transplantation. 2004; 78:574–579. PMID: 15446317.
Article3. Yazumi S, Yoshimoto T, Hisatsune H, Hasegawa K, Kida M, Tada S, et al. Endoscopic treatment of biliary complications after right-lobe living-donor liver transplantation with duct-to-duct biliary anastomosis. J Hepatobiliary Pancreat Surg. 2006; 13:502–510. PMID: 17139423.
Article4. Tsujino T, Isayama H, Sugawara Y, Sasaki T, Kogure H, Nakai Y, et al. Endoscopic management of biliary complications after adult living donor liver transplantation. Am J Gastroenterol. 2006; 101:2230–2236. PMID: 16952286.
Article5. Chok KS, Lo CM. Biliary complications in right lobe living donor liver transplantation. Hepatol Int. 2016; 10:553–558. PMID: 26932842.
Article6. Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011; 24:379–392. PMID: 21143651.
Article7. Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008; 14:759–769. PMID: 18508368.
Article8. Liu CL, Fan ST, Lo CM, Wei WI, Chan SC, Yong BH, et al. Operative outcomes of adult-to-adult right lobe live donor liver transplantation: a comparative study with cadaveric whole-graft liver transplantation in a single center. Ann Surg. 2006; 243:404–410. PMID: 16495707.
Article9. Gómez CM, Dumonceau JM, Marcolongo M, de Santibañes E, Ciardullo M, Pekolj J, et al. Endoscopic management of biliary complications after adult living-donor versus deceased-donor liver transplantation. Transplantation. 2009; 88:1280–1285. PMID: 19996927.
Article10. Samstein B, Smith AR, Freise CE, Zimmerman MA, Baker T, Olthoff KM, et al. Complications and their resolution in recipients of deceased and living donor liver transplants: findings from the A2ALL Cohort Study. Am J Transplant. 2016; 16:594–602. PMID: 26461803.
Article11. Kimura W. Pancreaticojejunal anastomosis, using a stent tube, in pancreaticoduodenectomy. J Hepatobiliary Pancreat Surg. 2009; 16:305–309. PMID: 19350197.
Article12. Azumi Y, Isaji S. Stented pancreaticojejunostomy (with video). J Hepatobiliary Pancreat Sci. 2012; 19:116–124. PMID: 22076668.
Article13. Levy MJ, Chari S, Adler DG, Clain JE, Gostout CJ, Harewood GC, et al. Complications of temporary pancreatic stent insertion for pancreaticojejunal anastomosis during pancreaticoduodenectomy. Gastrointest Endosc. 2004; 59:719–724. PMID: 15114323.
Article14. Zhou Y, Yang C, Wang S, Chen J, Li B. Does external pancreatic duct stent decrease pancreatic fistula rate after pancreatic resection?: a meta-analysis. Pancreatology. 2011; 11:362–370. PMID: 21876365.
Article15. Xiong JJ, Altaf K, Mukherjee R, Huang W, Hu WM, Li A, et al. Systematic review and meta-analysis of outcomes after intraoperative pancreatic duct stent placement during pancreaticoduodenectomy. Br J Surg. 2012; 99:1050–1061. PMID: 22622664.
Article16. Tashiro H, Ogawa T, Itamoto T, Ushitora Y, Tanimoto Y, Oshita A, et al. Synthetic bioabsorbable stent material for duct-to-duct biliary reconstruction. J Surg Res. 2009; 151:85–88. PMID: 18674784.
Article17. Tanimoto Y, Tashiro H, Mikuriya Y, Kuroda S, Hashimoto M, Kobayashi T, et al. Radiopaque biodegradable stent for duct-to-duct biliary reconstruction in pigs. Langenbecks Arch Surg. 2016; 401:513–517. PMID: 27138018.
Article18. Aikawa M, Miyazawa M, Okada K, Toshimitsu Y, Okamoto K, Akimoto N, et al. Development of a novel reflux-free bilioenteric anastomosis procedure by using a bioabsorbable polymer tube. J Hepatobiliary Pancreat Sci. 2010; 17:284–290. PMID: 19812888.
Article19. Itoi T, Kasuya K, Abe Y, Isayama H. Endoscopic placement of a new short-term biodegradable pancreatic and biliary stent in an animal model: a preliminary feasibility study (with videos). J Hepatobiliary Pancreat Sci. 2011; 18:463–467. PMID: 21170555.
Article20. Laukkarinen J, Sand J, Leppiniemi J, Kellomäki M, Nordback I. A novel technique for hepaticojejunostomy for nondilated bile ducts: a purse-string anastomosis with an intra-anastomotic biodegradable biliary stent. Am J Surg. 2010; 200:124–130. PMID: 20074696.
Article21. Shi J, Lv Y, Yu L, Zhang B, Zhang X, Fan C, et al. Interest of a new biodegradable stent coated with paclitaxel on anastomotic wound healing after biliary reconstruction. Eur J Gastroenterol Hepatol. 2013; 25:1415–1423. PMID: 23669325.
Article22. Kwon CI, Son JS, Kim KS, Moon JP, Park S, Jeon J, et al. Mechanical properties and degradation process of biliary self-expandable biodegradable stents. Dig Endosc. 2021; 33:1158–1169. PMID: 33319399.
Article23. Innes JT, Ferrara JJ, Carey LC. Biliary reconstruction without transanastomotic stent. Am Surg. 1988; 54:27–30. PMID: 3337479.24. Bismuth H, Franco D, Corlette MB, Hepp J. Long term results of Roux-en-Y hepaticojejunostomy. Surg Gynecol Obstet. 1978; 146:161–167. PMID: 622659.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vivo porcine study of 3D-printed biodegradable paclitaxel-eluting stent for biliary stricture after liver transplantation
- Biodegradable Stent/Tube for Pancreatic and Biliary Disease
- Biliary Endoprosthese by the Use of Expandable Metallic Stents
- Magnetic Compression Anastomosis for the Treatment of Post-Transplant Biliary Stricture
- Complex percutaneous biliary procedures: Review and contributions of a high volume team