J Stroke.
2022 Jan;24(1):88-97. 10.5853/jos.2020.04952.
Low Exposure to Direct Oral Anticoagulants Is Associated with Ischemic Stroke and Its Severity
- Affiliations
-
- 1Department of Neurology, University of Heidelberg, Im Neuenheimer Feld, Heidelberg, Germany
- 2Department of Clinical Pharmacology and Pharmacoepidemiology, University of Heidelberg, Im Neuenheimer Feld, Heidelberg, Germany
- 3Faculty of Health/School of Medicine, Witten/Herdecke University, Witten, Germany
- 4Department for Child and Adolescent Psychiatry, Johannes Gutenberg-University, Mainz, Germany
- KMID: 2525334
- DOI: http://doi.org/10.5853/jos.2020.04952
Abstract
- Background and purpose
In acute stroke patients, plasma concentrations of direct oral anticoagulants (DOAC) at hospital admission only poorly mirror DOAC exposure or the coagulation status at the time of the event. Here, we evaluated whether DOAC exposure and DOAC plasma concentration at the time of transient ischemic attacks (TIA) and ischemic strokes correlate with their likelihood of occurrence.
Methods
Prospectively, consecutive DOAC patients with acute ischemic stroke or TIA were included. Admission DOAC plasma concentrations were measured by ultraperformance liquid chromatography– tandem mass spectrometry. Individual DOAC exposure (area under the curve) and DOAC concentrations at event onset were derived from population pharmacokinetic analyses.
Results
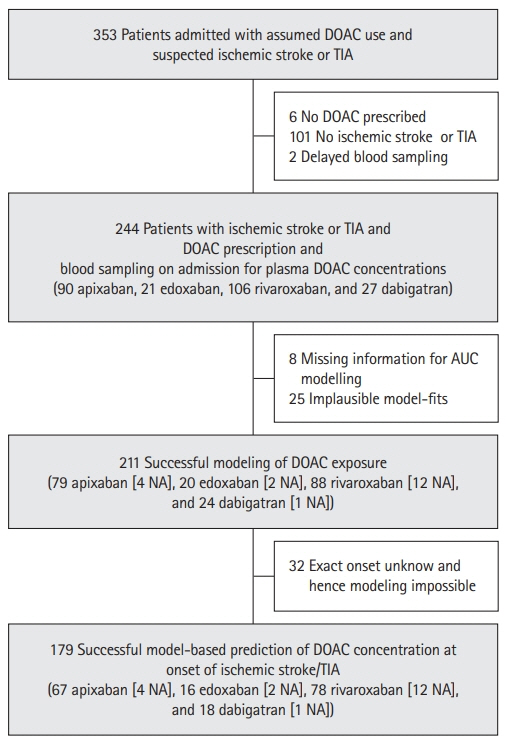
DOAC exposure was successfully modeled in 211 patients (ischemic stroke 74.4%, TIA 25.6%). Compared to published values, 63.0% had relatively lower DOAC exposure and they more often received lower DOAC doses than recommended (odds ratio [OR], 2.125; 95% confidence interval [CI], 1.039 to 4.560; P=0.044). These patients more likely suffered ischemic strokes than TIA (OR, 2.411; 95% CI, 1.254 to 4.638; P=0.008) and their strokes were more severe (slope, 3.161; 95% CI, 0.741 to 5.58; P=0.011). Low relative DOAC concentrations at event onset were likewise associated with ischemic strokes (OR, 4.123; 95% CI, 1.834 to 9.268; P=0.001), but not to stroke severity (P=0.272). DOAC exposure had a higher explanatory value for stroke severity than concentrations at event.
Conclusions
Low DOAC exposure is strongly associated to ischemic stroke and its severity. By monitoring DOAC plasma concentrations, patients prone to ischemic stroke might be identified.
Keyword
Figure
Cited by 1 articles
-
Association Between Plasma Anti-Factor Xa Concentrations and Large Artery Occlusion in Patients With Acute Ischemic Stroke Taking Direct Oral Anticoagulants for Non-valvular Atrial Fibrillation
Dae-Hyun Kim, Byung-Cheol Kwak, Byeol-A Yoon, Jae-Kwan Cha, Jong-Sung Park, Min-Sun Kwak, Kwang-Sook Woo, Jin-Yeong Han
Ann Lab Med. 2024;44(5):459-462. doi: 10.3343/alm.2024.0036.
Reference
-
References
1. Purrucker JC, Steiner T. Management of acute stroke in patients on oral anticoagulants. Curr Opin Neurol. 2017; 30:1–7.
Article2. Purrucker JC, Haas K, Rizos T, Khan S, Poli S, Kraft P, et al. Coagulation testing in acute ischemic stroke patients taking non-vitamin k antagonist oral anticoagulants. Stroke. 2017; 48:152–158.
Article3. Seiffge DJ, Traenka C, Polymeris A, Hert L, Fisch U, Peters N, et al. Feasibility of rapid measurement of rivaroxaban plasma levels in patients with acute stroke. J Thromb Thrombolysis. 2017; 43:112–116.
Article4. Volbers B, Köhrmann M, Kallmünzer B, Kurka N, Breuer L, Ringwald J, et al. Dabigatran plasma levels in acute cerebrovascular events. J Stroke Cerebrovasc Dis. 2016; 25:877–882.
Article5. Macha K, Marsch A, Siedler G, Breuer L, Strasser EF, Engelhorn T, et al. Cerebral ischemia in patients on direct oral anticoagulants. Stroke. 2019; 50:873–879.
Article6. Seiffge DJ, Kägi G, Michel P, Fischer U, Béjot Y, Wegener S, et al. Rivaroxaban plasma levels in acute ischemic stroke and intracerebral hemorrhage. Ann Neurol. 2018; 83:451–459.
Article7. Gosselin RC, Adcock DM, Bates SM, Douxfils J, Favaloro EJ, Gouin-Thibault I, et al. International Council for Standardization in Haematology (ICSH) recommendations for laboratory measurement of direct oral anticoagulants. Thromb Haemost. 2018; 118:437–450.
Article8. Foerster KI, Huppertz A, Müller OJ, Rizos T, Tilemann L, Haefeli WE, et al. Simultaneous quantification of direct oral anticoagulants currently used in anticoagulation therapy. J Pharm Biomed Anal. 2018; 148:238–244.
Article9. Byon W, Sweeney K, Frost C, Boyd RA. Population pharmacokinetics, pharmacodynamics, and exploratory exposure-response analyses of apixaban in subjects treated for venous thromboembolism. CPT Pharmacometrics Syst Pharmacol. 2017; 6:340–349.
Article10. Liesenfeld KH, Lehr T, Dansirikul C, Reilly PA, Connolly SJ, Ezekowitz MD, et al. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost. 2011; 9:2168–2175.
Article11. Krekels EH, Niebecker R, Karlsson MO, Miller R, Shimizu T, Karlsson KE, et al. Population pharmacokinetics of edoxaban in patients with non-valvular atrial fibrillation in the ENGAGE AF-TIMI 48 study, a phase III clinical trial. Clin Pharmacokinet. 2016; 55:1079–1090.
Article12. Girgis IG, Patel MR, Peters GR, Moore KT, Mahaffey KW, Nessel CC, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with non-valvular atrial fibrillation: results from ROCKET AF. J Clin Pharmacol. 2014; 54:917–927.
Article13. Cirincione B, Kowalski K, Nielsen J, Roy A, Thanneer N, Byon W, et al. Population pharmacokinetics of apixaban in subjects with nonvalvular atrial fibrillation. CPT Pharmacometrics Syst Pharmacol. 2018; 7:728–738.
Article14. Stangier J. Clinical pharmacokinetics and pharmacodynamics of the oral direct thrombin inhibitor dabigatran etexilate. Clin Pharmacokinet. 2008; 47:285–295.15. Yin OQ, Tetsuya K, Miller R. Edoxaban population pharmacokinetics and exposure-response analysis in patients with non-valvular atrial fibrillation. Eur J Clin Pharmacol. 2014; 70:1339–1351.
Article16. Assessment report Xarelto VHF. European Medicines Agency;https://www.ema.europa.eu/en/documents/variation-report/xarelto-h-c-944-ii-0012-epar-assessment-report-variation_en.pdf. 2012. Accessed November 24, 2021.17. Yeo IK, Johnson RA. A new family of power transformations to improve normality or symmetry. Biometrika. 2000; 87:954–959.
Article18. Vuong QH. Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica. 1989; 57:307–333.
Article19. Wang X, Tirucherai G, Marbury TC, Wang J, Chang M, Zhang D, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol. 2016; 56:628–636.
Article20. Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939–an oral, direct Factor Xa inhibitor: after multiple dosing in healthy male subjects. Eur J Clin Pharmacol. 2005; 61:873–880.21. Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol. 2014; 63:321–328.22. Ruff CT, Giugliano RP, Braunwald E, Morrow DA, Murphy SA, Kuder JF, et al. Association between edoxaban dose, concentration, anti-factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet. 2015; 385:2288–2295.
Article23. Steinberg BA, Shrader P, Pieper K, Thomas L, Allen LA, Ansell J, et al. Frequency and outcomes of reduced dose non-vitamin K antagonist anticoagulants: results from ORBIT-AF II (The Outcomes Registry for Better Informed Treatment of Atrial Fibrillation II). J Am Heart Assoc. 2018; 7:e007633.
Article24. Borne RT, O‘Donnell C, Turakhia MP, Varosy PD, Jackevicius CA, Marzec LN, et al. Adherence and outcomes to direct oral anticoagulants among patients with atrial fibrillation: findings from the veterans health administration. BMC Cardiovasc Disord. 2017; 17:236.
Article25. Stoll S, Macha K, Marsch A, Gerner ST, Siedler G, Fröhlich K, et al. Ischemic stroke and dose adjustment of oral Factor Xa inhibitors in patients with atrial fibrillation. J Neurol. 2020; 267:2007–2012.
Article26. Woo HG, Chung I, Gwak DS, Kim BK, Kim BJ, Bae HJ, et al. Recurrent ischemic stroke in atrial fibrillation with non-vitamin K antagonist oral anticoagulation. J Clin Neurosci. 2019; 64:127–133.
Article27. Herink MC, Zhuo YF, Williams CD, DeLoughery TG. Clinical management of pharmacokinetic drug interactions with direct oral anticoagulants (DOACs). Drugs. 2019; 79:1625–1634.
Article28. Leil TA, Feng Y, Zhang L, Paccaly A, Mohan P, Pfister M. Quantification of apixaban’s therapeutic utility in prevention of venous thromboembolism: selection of phase III trial dose. Clin Pharmacol Ther. 2010; 88:375–382.
Article29. Sakaguchi T, Osanai H, Murase Y, Ishii H, Nakashima Y, Asano H, et al. Monitoring of anti-Xa activity and factors related to bleeding events: a study in Japanese patients with nonvalvular atrial fibrillation receiving rivaroxaban. J Cardiol. 2017; 70:244–249.
Article30. Hylek EM, Go AS, Chang Y, Jensvold NG, Henault LE, Selby JV, et al. Effect of intensity of oral anticoagulation on stroke severity and mortality in atrial fibrillation. N Engl J Med. 2003; 349:1019–1026.
Article31. Gallagher AM, Setakis E, Plumb JM, Clemens A, van Staa TP. Risks of stroke and mortality associated with suboptimal anticoagulation in atrial fibrillation patients. Thromb Haemost. 2011; 106:968–977.
Article32. Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, et al. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008; 118:2029–2037.
Article33. Ay H, Arsava EM, Gungor L, Greer D, Singhal AB, Furie KL, et al. Admission international normalized ratio and acute infarct volume in ischemic stroke. Ann Neurol. 2008; 64:499–506.
Article34. Nakamura A, Ago T, Kamouchi M, Hata J, Matsuo R, Kuroda J, et al. Intensity of anticoagulation and clinical outcomes in acute cardioembolic stroke: the Fukuoka Stroke Registry. Stroke. 2013; 44:3239–3242.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Practical Issues to Prevent Stroke Associated with Non-valvular Atrial Fibrillation
- Non-Vitamin K Oral Anticoagulants in Stroke Patients: Practical Issues
- Non-Vitamin K Oral Anticoagulants in Stroke Patients: Practical Issues
- Use of Non-Vitamin K Dependent Oral Anticoagulant in Ischemic Stroke
- Pharmacological Secondary Prevention of Ischemic Stroke