Brain Tumor Res Treat.
2022 Jan;10(1):61-67. 10.14791/btrt.2022.10.e29.
Reverse Trans-Sellar Neuroendoscopic Management of a Large Rathke’s Cleft Cyst Causing Obstructive Hydrocephalus: A Case Report
- Affiliations
-
- 1Department of Neurosurgery, Chungnam National University Hospital, Chungnam National University School of Medicine, Daejeon, Korea
- 2Department of Neurosurgery, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2525295
- DOI: http://doi.org/10.14791/btrt.2022.10.e29
Abstract
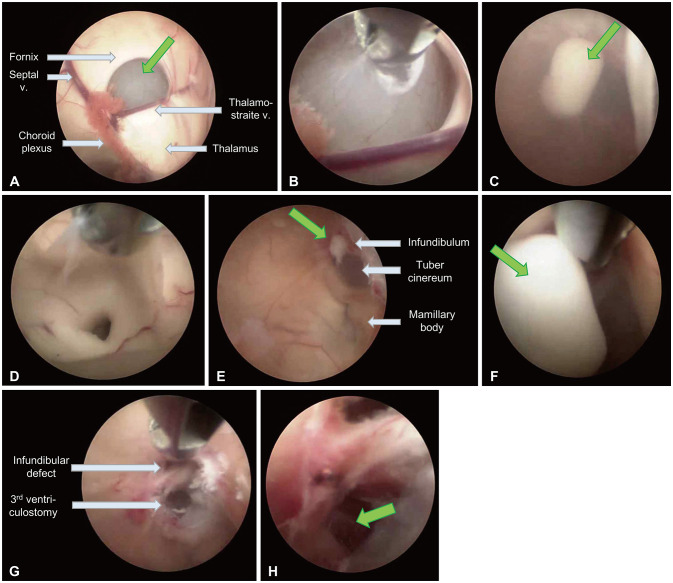
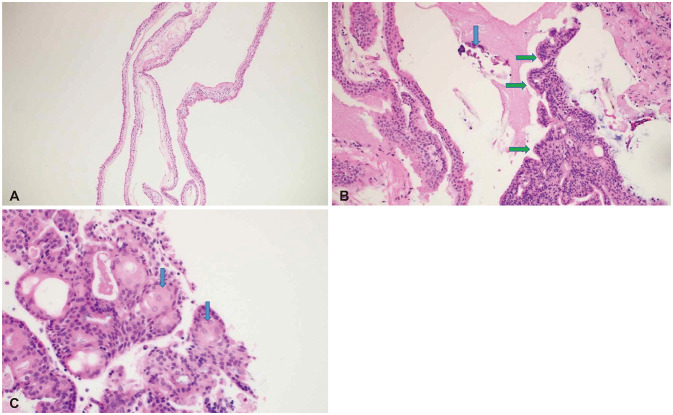
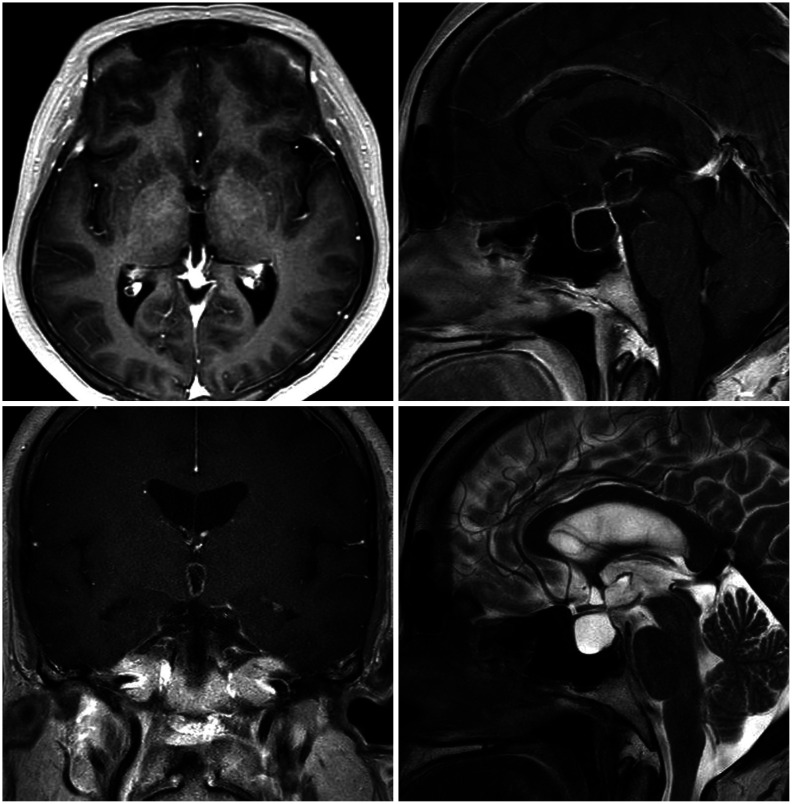
- Symptomatic Rathke’s cleft cysts (RCCs) can be treated by surgical procedures, usually through an endonasal transsphenoidal corridor using either a microscope or an endoscope. We report a large suprasellar extended RCC causing obstructive hydrocephalus, which was efficiently managed by a novel surgical route named “reverse” trans-sellar approach using transventricular neuroendoscopy. A 48-yearold woman complained of persistent headache and a tendency to fall that had begun 6 months previously. The images obtained from MRI scan showed intra- and supra-sellar cystic masses occupying the third ventricle with obstruction of the foramina of Monro and the aqueduct of Sylvius. The cystic wall showed a slight enhancement, and the cystic contents showed iso-signal intensity on T1-and T2-weighted images. Instead of trans-nasal trans-sellar surgery, we decided to operate using a conventional transventricular endoscope. A thin cystic capsule, which blocked the foramina of Monro and the aqueduct of Sylvius, was fenestrated and removed and a third ventriculostomy was performed. The defect in the infundibulum between sellar and suprasellar cysts was widened and used as a corridor to drain cystic contents (reverse trans-sellar route). The final pathological finding revealed an RCC with focal metaplasia. We efficiently managed a large RCC by transventricular neuroendoscopic surgery with cyst fenestration and third ventriculostomy and simultaneously drained the sellar contents using a novel surgical route. Reverse trans-sellar neuroendoscopic surgery is a relevant treatment option for selective patients with large suprasellar extensions of RCCs.
Figure
Reference
-
1. Barrow DL, Spector RH, Takei Y, Tindall GT. Symptomatic Rathke’s cleft cysts located entirely in the suprasellar region: review of diagnosis, management, and pathogenesis. Neurosurgery. 1985; 16:766–772. PMID: 4010898.2. Rottenberg GT, Chong WK, Powell M, Kendall BE. Cyst formation of the craniopharyngeal duct. Clin Radiol. 1994; 49:126–129. PMID: 8124891.3. Voelker JL, Campbell RL, Muller J. Clinical, radiographic, and pathological features of symptomatic Rathke’s cleft cysts. J Neurosurg. 1991; 74:535–544. PMID: 2002366.4. Larkin S, Karavitaki N, Ansorge O. Rathke’s cleft cyst. Handb Clin Neurol. 2014; 124:255–269. PMID: 25248592.
Article5. Han SJ, Rolston JD, Jahangiri A, Aghi MK. Rathke’s cleft cysts: review of natural history and surgical outcomes. J Neurooncol. 2014; 117:197–203. PMID: 24146189.6. Kino H, Akutsu H, Tanaka S, Hara T, Miyamoto H, Sakamoto N, et al. Endoscopic endonasal cyst fenestration into the sphenoid sinus using the mucosa coupling method for symptomatic Rathke’s cleft cyst: a novel method for maintaining cyst drainage to prevent recurrence. J Neurosurg. 2020; 133:1710–1720.7. Higgins DM, Van Gompel JJ, Nippoldt TB, Meyer FB. Symptomatic Rathke cleft cysts: extent of resection and surgical complications. Neurosurg Focus. 2011; 31:E2.8. Jahangiri A, Potts M, Kunwar S, Blevins L, El-Sayed IH, Aghi MK. Extended endoscopic endonasal approach for suprasellar Rathke’s cleft cysts. J Clin Neurosci. 2014; 21:779–785. PMID: 24308953.9. Trifanescu R, Ansorge O, Wass JA, Grossman AB, Karavitaki N. Rathke’s cleft cysts. Clin Endocrinol (Oxf). 2012; 76:151–160. PMID: 21951110.10. Osborn AG, Preece MT. Intracranial cysts: radiologic-pathologic correlation and imaging approach. Radiology. 2006; 239:650–664. PMID: 16714456.11. Famini P, Maya MM, Melmed S. Pituitary magnetic resonance imaging for sellar and parasellar masses: ten-year experience in 2598 patients. J Clin Endocrinol Metab. 2011; 96:1633–1641. PMID: 21470998.12. Kim JE, Kim JH, Kim OL, Paek SH, Kim DG, Chi JG, et al. Surgical treatment of symptomatic Rathke cleft cysts: clinical features and results with special attention to recurrence. J Neurosurg. 2004; 100:33–40. PMID: 14743909.13. Wen L, Hu LB, Feng XY, Desai G, Zou LG, Wang WX, et al. Rathke’s cleft cyst: clinicopathological and MRI findings in 22 patients. Clin Radiol. 2010; 65:47–55. PMID: 20103421.14. Benveniste RJ, King WA, Walsh J, Lee JS, Naidich TP, Post KD. Surgery for Rathke cleft cysts: technical considerations and outcomes. J Neurosurg. 2004; 101:577–584. PMID: 15481709.15. Shin JL, Asa SL, Woodhouse LJ, Smyth HS, Ezzat S. Cystic lesions of the pituitary: clinicopathological features distinguishing craniopharyngioma, Rathke’s cleft cyst, and arachnoid cyst. J Clin Endocrinol Metab. 1999; 84:3972–3982. PMID: 10566636.16. Isono M, Kamida T, Kobayashi H, Shimomura T, Matsuyama J. Clinical features of symptomatic Rathke’s cleft cyst. Clin Neurol Neurosurg. 2001; 103:96–100. PMID: 11516552.17. Nakahara Y, Koga H, Maeda K, Takagi M, Tabuchi K. Neuroendoscopic transventricular surgery for suprasellar cystic mass lesions such as cystic craniopharyngioma and Rathke cleft cyst. Neurol Med Chir (Tokyo). 2004; 44:408–413. discussion 414-5. PMID: 15508348.18. Aho CJ, Liu C, Zelman V, Couldwell WT, Weiss MH. Surgical outcomes in 118 patients with Rathke cleft cysts. J Neurosurg. 2005; 102:189–193.19. Mendelson ZS, Husain Q, Elmoursi S, Svider PF, Eloy JA, Liu JK. Rathke’s cleft cyst recurrence after transsphenoidal surgery: a meta-analysis of 1151 cases. J Clin Neurosci. 2014; 21:378–385. PMID: 24269553.20. Xie T, Hu F, Yu Y, Gu Y, Wang X, Zhang X. Endoscopic endonasal resection of symptomatic Rathke cleft cysts. J Clin Neurosci. 2011; 18:760–762. PMID: 21493070.21. Frank G, Sciarretta V, Mazzatenta D, Farneti G, Modugno GC, Pasquini E. Transsphenoidal endoscopic approach in the treatment of Rathke’s cleft cyst. Neurosurgery. 2005; 56:124–128. discussion 129. PMID: 15617594.22. Madhok R, Prevedello DM, Gardner P, Carrau RL, Snyderman CH, Kassam AB. Endoscopic endonasal resection of Rathke cleft cysts: clinical outcomes and surgical nuances. J Neurosurg. 2010; 112:1333–1339. PMID: 19929190.23. Hayashi Y, Kobayashi M, Sasagawa Y, Oishi M, Tachibana O, Nakada M. Entirely suprasellar Rathke cleft cysts: clinical features and surgical efficacy of endoscopic endonasal transtuberculum sellae approach. World Neurosurg. 2019; 126:e921–e929. PMID: 30872194.24. Peng Y, Fan J, Li Y, Qiu M, Qi S. The supraorbital keyhole approach to the suprasellar and supra-intrasellar Rathke cleft cysts under pure endoscopic visualization. World Neurosurg. 2016; 92:120–125. PMID: 27163551.25. Ogawa Y, Watanabe M, Tominaga T. Spontaneous alteration from Rathke’s cleft cyst to craniopharyngioma--possible involvement of transformation between these pathologies. Endocr Pathol. 2014; 25:422–426. PMID: 25154633.26. Manjila S, Asmar NE, Vidalis BM, Alonso F, Singh G, Vadamalai K, et al. Intratumoral Rathke’s cleft cyst remnants within craniopharyngioma, pituitary adenoma, suprasellar dermoid, and epidermoid cysts: a ubiquitous signature of ectodermal lineage or a transitional entity? Neurosurgery. 2019; 85:180–188. PMID: 30010935.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Suprasellar Rathke Cleft Cyst: A case report
- Large Ossified Rathke's Cleft Cyst: A Case Report and Review of the Literature
- Symptomatic Rathke's Cleft Cyst in the Interpeduncular Cistern: Case Report
- Rathke's Cleft Cyst: Case Report
- Suprasellar Chordoid Glioma Combined with Rathke's Cleft Cyst: Case Report