Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- KMID: 2525122
- DOI: http://doi.org/10.4093/dmj.2021.0335
Abstract
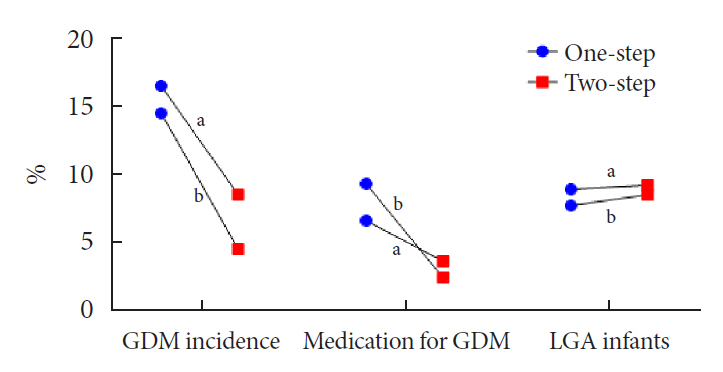
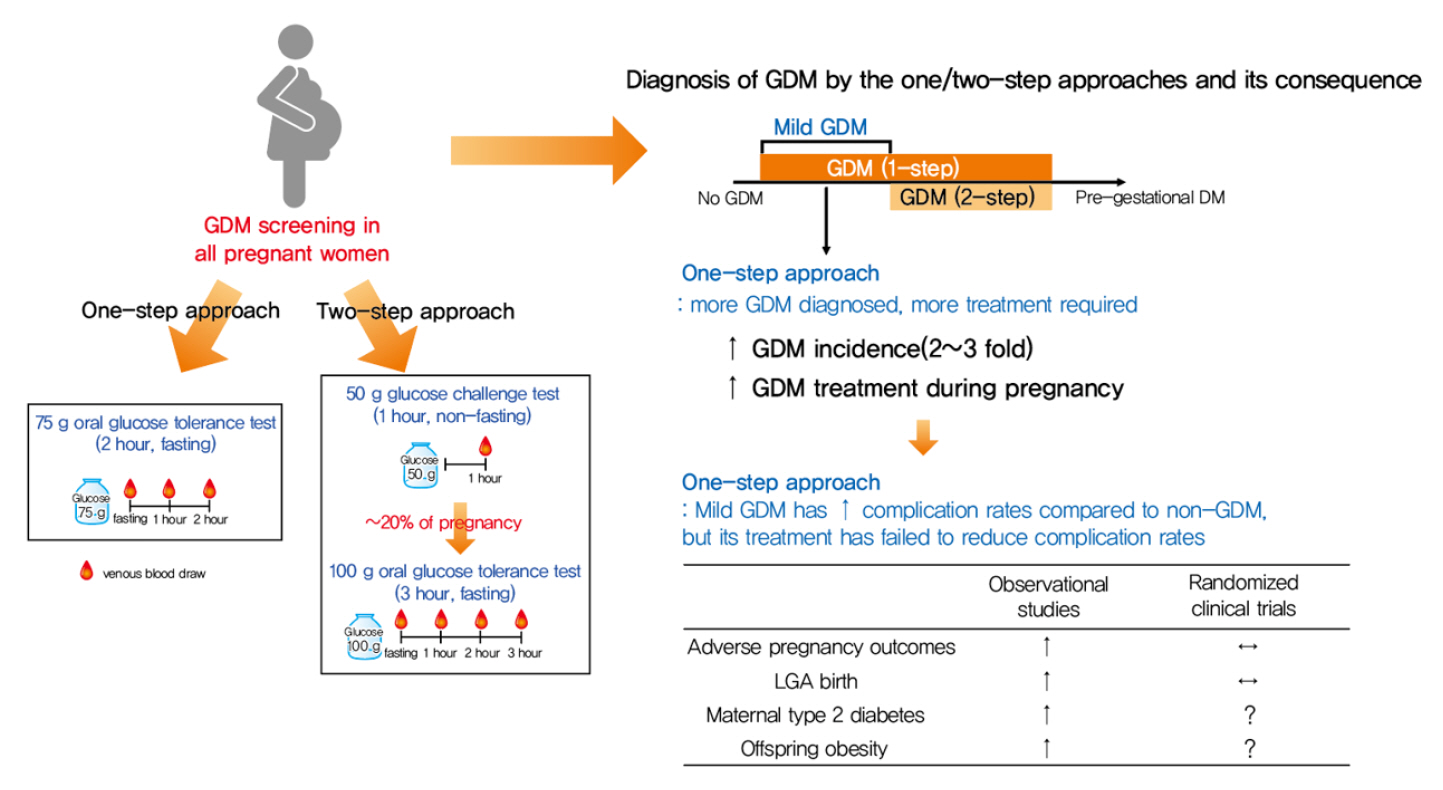
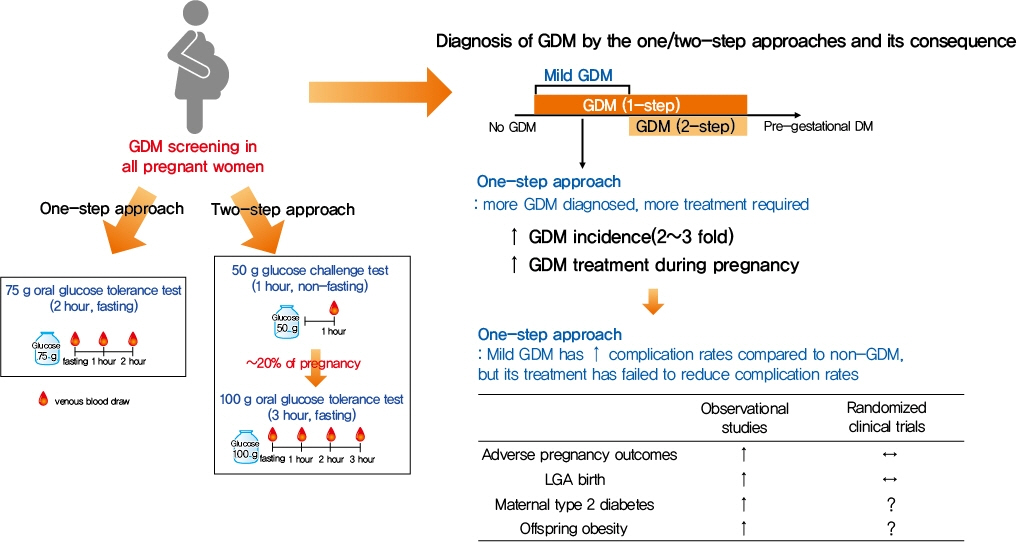
- Gestational diabetes mellitus (GDM) is the most common complication during pregnancy and is defined as any degree of glucose intolerance with onset or first recognition during pregnancy. GDM is associated with adverse pregnancy outcomes and long-term offspring and maternal complications. For GDM screening and diagnosis, a two-step approach (1-hour 50 g glucose challenge test followed by 3-hour 100 g oral glucose tolerance test) has been widely used. After the Hyperglycemia and Adverse Pregnancy Outcome study implemented a 75 g oral glucose tolerance test in all pregnant women, a one-step approach was recommended as an option for the diagnosis of GDM after 2010. The one-step approach has more than doubled the incidence of GDM, but its clinical benefit in reducing adverse pregnancy outcomes remains controversial. Long-term complications of mothers with GDM include type 2 diabetes mellitus and cardiovascular disease, and complications of their offspring include childhood obesity and glucose intolerance. The diagnostic criteria of GDM should properly classify women at risk for adverse pregnancy outcomes and long-term complications. The present review summarizes the strengths and weaknesses of the one-step and two-step approaches for the diagnosis of GDM based on recent randomized controlled trials and observational studies. We also describe the long-term maternal and offspring complications of GDM.
Figure
Cited by 3 articles
-
Higher Muscle Mass Protects Women with Gestational Diabetes Mellitus from Progression to Type 2 Diabetes Mellitus
Yujin Shin, Joon Ho Moon, Tae Jung Oh, Chang Ho Ahn, Jae Hoon Moon, Sung Hee Choi, Hak Chul Jang
Diabetes Metab J. 2022;46(6):890-900. doi: 10.4093/dmj.2021.0334.2023 Clinical Practice Guidelines for Diabetes Management in Korea: Full Version Recommendation of the Korean Diabetes Association
Jun Sung Moon, Shinae Kang, Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, Yoon Ju Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae Jin Kim, Hyun Min Kim, Jung Hae Ko, Nam Hoon Kim, Chong Hwa Kim, Jeeyun Ahn, Tae Jung Oh, Soo-Kyung Kim, Jaehyun Kim, Eugene Han, Sang-Man Jin, Jaehyun Bae, Eonju Jeon, Ji Min Kim, Seon Mee Kang, Jung Hwan Park, Jae-Seung Yun, Bong-Soo Cha, Min Kyong Moon, Byung-Wan Lee
Diabetes Metab J. 2024;48(4):546-708. doi: 10.4093/dmj.2024.0249.Amelioration of Insulin Resistance after Delivery Is Associated with Reduced Risk of Postpartum Diabetes in Women with Gestational Diabetes Mellitus
Heejun Son, Joon Ho Moon, Sung Hee Choi, Nam H. Cho, Soo Heon Kwak, Hak Chul Jang
Endocrinol Metab. 2024;39(5):701-710. doi: 10.3803/EnM.2024.1974.
Reference
-
1. Guariguata L, Linnenkamp U, Beagley J, Whiting DR, Cho NH. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res Clin Pract. 2014; 103:176–85.
Article2. Kim KS, Hong S, Han K, Park CY. The clinical characteristics of gestational diabetes mellitus in Korea: a National Health Information Database Study. Endocrinol Metab (Seoul). 2021; 36:628–36.
Article3. Metzger BE. Summary and recommendations of the Third International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes. 1991; 40 Suppl 2:197–201.
Article4. Jang HC, Cho NH, Min YK, Han IK, Jung KB, Metzger BE. Increased macrosomia and perinatal morbidity independent of maternal obesity and advanced age in Korean women with GDM. Diabetes Care. 1997; 20:1582–8.
Article5. HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008; 358:1991–2002.
Article6. Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009; 361:1339–48.
Article7. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005; 352:2477–86.
Article8. Lowe WL Jr, Lowe LP, Kuang A, Catalano PM, Nodzenski M, Talbot O, et al. Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Diabetologia. 2019; 62:598–610.
Article9. Kim C. Management of cardiovascular risk in perimenopausal women with diabetes. Diabetes Metab J. 2021; 45:492–501.
Article10. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002; 25:1862–8.11. Parikh NI, Gonzalez JM, Anderson CA, Judd SE, Rexrode KM, Hlatky MA, et al. Adverse pregnancy outcomes and cardiovascular disease risk: unique opportunities for cardiovascular disease prevention in women: a scientific statement from the American Heart Association. Circulation. 2021; 143:e902–16.
Article12. O’Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964; 13:278–85.13. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982; 144:768–73.
Article14. International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010; 33:676–82.
Article15. O’Sullivan JB, Mahan CM, Charles D, Dandrow RV. Screening criteria for high-risk gestational diabetic patients. Am J Obstet Gynecol. 1973; 116:895–900.
Article16. Donovan L, Hartling L, Muise M, Guthrie A, Vandermeer B, Dryden DM. Screening tests for gestational diabetes: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013; 159:115–22.
Article17. National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979; 28:1039–57.18. Gabbe SG. Management of diabetes mellitus in pregnancy. Am J Obstet Gynecol. 1985; 153:824–8.
Article19. Jang HC, Cho NH, Jung KB, Oh KS, Dooley SL, Metzger BE. Screening for gestational diabetes mellitus in Korea. Int J Gynaecol Obstet. 1995; 51:115–22.
Article20. Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care. 2012; 35:526–8.21. Hillier TA, Pedula KL, Ogasawara KK, Vesco KK, Oshiro CE, Lubarsky SL, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021; 384:895–904.
Article22. Davis EM, Abebe KZ, Simhan HN, Catalano P, Costacou T, Comer D, et al. Perinatal outcomes of two screening strategies for gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol. 2021; 138:6–15.
Article23. Kim MH, Kwak SH, Kim SH, Hong JS, Chung HR, Choi SH, et al. Pregnancy outcomes of women additionally diagnosed as gestational diabetes by the International Association of the Diabetes and Pregnancy study groups criteria. Diabetes Metab J. 2019; 43:766–75.
Article24. Caissutti C, Khalifeh A, Saccone G, Berghella V. Are women positive for the one step but negative for the two step screening tests for gestational diabetes at higher risk for adverse outcomes? Acta Obstet Gynecol Scand. 2018; 97:122–34.
Article25. Duran A, Saenz S, Torrejon MJ, Bordiu E, Del Valle L, Galindo M, et al. Introduction of IADPSG criteria for the screening and diagnosis of gestational diabetes mellitus results in improved pregnancy outcomes at a lower cost in a large cohort of pregnant women: the St. Carlos Gestational Diabetes Study. Diabetes Care. 2014; 37:2442–50.26. Pocobelli G, Yu O, Fuller S, Fraser JR, Wartko PD, Chen L, et al. One-step approach to identifying gestational diabetes mellitus: association with perinatal outcomes. Obstet Gynecol. 2018; 132:859–67.27. Freinkel N. Banting lecture 1980. Of pregnancy and progeny. Diabetes. 1980; 29:1023–35.
Article28. Kim W, Park SK, Kim YL. Fetal abdominal obesity detected at 24 to 28 weeks of gestation persists until delivery despite management of gestational diabetes mellitus. Diabetes Metab J. 2021; 45:547–57.
Article29. Han IK, Min HK, Yim CH, Jeong HY, Chang HC, Han KO, et al. Prediction of large for gestational age infant in women with gestational age infant in women with gestational diabetes mellitus by ultrasound examination. J Korean Diabetes Assoc. 1999; 23:326–35.30. Kim M, Park J, Kim SH, Kim YM, Yee C, Choi SJ, et al. The trends and risk factors to predict adverse outcomes in gestational diabetes mellitus: a 10-year experience from 2006 to 2015 in a single tertiary center. Obstet Gynecol Sci. 2018; 61:309–18.
Article31. Silva JK, Kaholokula JK, Ratner R, Mau M. Ethnic differences in perinatal outcome of gestational diabetes mellitus. Diabetes Care. 2006; 29:2058–63.
Article32. ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018; 131:e49–64.33. Ahmed B, Abushama M, Khraisheh M, Dudenhausen J. Role of ultrasound in the management of diabetes in pregnancy. J Matern Fetal Neonatal Med. 2015; 28:1856–63.
Article34. Lowe WL Jr, Scholtens DM, Kuang A, Linder B, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019; 42:372–80.35. Scholtens DM, Kuang A, Lowe LP, Hamilton J, Lawrence JM, Lebenthal Y, et al. Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study (HAPO FUS): maternal glycemia and childhood glucose metabolism. Diabetes Care. 2019; 42:381–92.36. Chung HR, Moon JH, Lim JS, Lee YA, Shin CH, Hong JS, et al. Maternal hyperglycemia during pregnancy increases adiposity of offspring. Diabetes Metab J. 2021; 45:730–8.
Article37. Tam WH, Ma RC, Ozaki R, Li AM, Chan MH, Yuen LY, et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care. 2017; 40:679–86.
Article38. Kim E, Kwak SH, Chung HR, Ohn JH, Bae JH, Choi SH, et al. DNA methylation profiles in sibling pairs discordant for intrauterine exposure to maternal gestational diabetes. Epigenetics. 2017; 12:825–32.
Article39. Shokry E, Marchioro L, Uhl O, Bermudez MG, Garcia-Santos JA, Segura MT, et al. Impact of maternal BMI and gestational diabetes mellitus on maternal and cord blood metabolome: results from the PREOBE cohort study. Acta Diabetol. 2019; 56:421–30.
Article40. Liu F, Zhao C, Liu L, Ding H, Huo R, Shi Z. Peptidome profiling of umbilical cord plasma associated with gestational diabetes-induced fetal macrosomia. J Proteomics. 2016; 139:38–44.
Article41. Cheng X, Chapple SJ, Patel B, Puszyk W, Sugden D, Yin X, et al. Gestational diabetes mellitus impairs Nrf2-mediated adaptive antioxidant defenses and redox signaling in fetal endothelial cells in utero. Diabetes. 2013; 62:4088–97.
Article42. Jang HC. Gestational diabetes in Korea: incidence and risk factors of diabetes in women with previous gestational diabetes. Diabetes Metab J. 2011; 35:1–7.
Article43. Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009; 373:1773–9.
Article44. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020; 369:m1361.
Article45. Kwak SH, Choi SH, Jung HS, Cho YM, Lim S, Cho NH, et al. Clinical and genetic risk factors for type 2 diabetes at early or late post partum after gestational diabetes mellitus. J Clin Endocrinol Metab. 2013; 98:E744–52.
Article46. Moon JH, Kwak SH, Jang HC. Prevention of type 2 diabetes mellitus in women with previous gestational diabetes mellitus. Korean J Intern Med. 2017; 32:26–41.
Article47. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021; 44(Suppl 1):S15–33.48. Oh TJ, Kim YG, Kang S, Moon JH, Kwak SH, Choi SH, et al. Oral glucose tolerance testing allows better prediction of diabetes in women with a history of gestational diabetes mellitus. Diabetes Metab J. 2019; 43:342–9.
Article49. Kwak SH, Choi SH, Kim K, Jung HS, Cho YM, Lim S, et al. Prediction of type 2 diabetes in women with a history of gestational diabetes using a genetic risk score. Diabetologia. 2013; 56:2556–63.
Article50. Ignell C, Shaat N, Ekelund M, Berntorp K. The impact of ethnicity on glucose homeostasis after gestational diabetes mellitus. Acta Diabetol. 2013; 50:927–34.
Article51. Kousta E, Efstathiadou Z, Lawrence NJ, Jeffs JA, Godsland IF, Barrett SC, et al. The impact of ethnicity on glucose regulation and the metabolic syndrome following gestational diabetes. Diabetologia. 2006; 49:36–40.
Article52. Cho YM, Kim TH, Lim S, Choi SH, Shin HD, Lee HK, et al. Type 2 diabetes-associated genetic variants discovered in the recent genome-wide association studies are related to gestational diabetes mellitus in the Korean population. Diabetologia. 2009; 52:253–61.
Article53. Bao W, Tobias DK, Bowers K, Chavarro J, Vaag A, Grunnet LG, et al. Physical activity and sedentary behaviors associated with risk of progression from gestational diabetes mellitus to type 2 diabetes mellitus: a prospective cohort study. JAMA Intern Med. 2014; 174:1047–55.
Article54. Moon JH, Kwak SH, Jung HS, Choi SH, Lim S, Cho YM, et al. Weight gain and progression to type 2 diabetes in women with a history of gestational diabetes mellitus. J Clin Endocrinol Metab. 2015; 100:3548–55.
Article55. Cho NH, Jang HC, Park HK, Cho YW. Waist circumference is the key risk factor for diabetes in Korean women with history of gestational diabetes. Diabetes Res Clin Pract. 2006; 71:177–83.
Article56. Moon JH, Kim H, Kim H, Park J, Choi W, Choi W, et al. Lactation improves pancreatic β cell mass and function through serotonin production. Sci Transl Med. 2020; 12:eaay0455.
Article57. Retnakaran R, Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. CMAJ. 2009; 181:371–6.
Article58. Tranidou A, Dagklis T, Tsakiridis I, Siargkas A, Apostolopoulou A, Mamopoulos A, et al. Risk of developing metabolic syndrome after gestational diabetes mellitus: a systematic review and meta-analysis. J Endocrinol Invest. 2021; 44:1139–49.
Article59. Xu Y, Shen S, Sun L, Yang H, Jin B, Cao X. Metabolic syndrome risk after gestational diabetes: a systematic review and meta-analysis. PLoS One. 2014; 9:e87863.
Article60. Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019; 139:1069–79.
Article61. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019; 62:905–14.
Article62. Gunderson EP, Quesenberry CP Jr, Jacobs DR Jr, Feng J, Lewis CE, Sidney S. Longitudinal study of prepregnancy cardiometabolic risk factors and subsequent risk of gestational diabetes mellitus: the CARDIA study. Am J Epidemiol. 2010; 172:1131–43.
Article63. Noussitou P, Monbaron D, Vial Y, Gaillard RC, Ruiz J. Gestational diabetes mellitus and the risk of metabolic syndrome: a population-based study in Lausanne, Switzerland. Diabetes Metab. 2005; 31(4 Pt 1):361–9.
Article64. Catov JM, Sun B, Bertolet M, Snyder GG, Lewis CE, Allen NB, et al. Changes in cardiometabolic risk factors before and after gestational diabetes: a prospective life-course analysis in CARDIA women. Obesity (Silver Spring). 2020; 28:1397–404.
Article65. Cho NH, Ahn CH, Moon JH, Kwak SH, Choi SH, Lim S, et al. Metabolic syndrome independently predicts future diabetes in women with a history of gestational diabetes mellitus. Medicine (Baltimore). 2016; 95:e4582.
Article66. Gunderson EP, Chiang V, Pletcher MJ, Jacobs DR, Quesenberry CP, Sidney S, et al. History of gestational diabetes mellitus and future risk of atherosclerosis in mid-life: the Coronary Artery Risk Development in Young Adults study. J Am Heart Assoc. 2014; 3:e000490.
Article67. Gunderson EP, Sun B, Catov JM, Carnethon M, Lewis CE, Allen NB, et al. Gestational diabetes history and glucose tolerance after pregnancy associated with coronary artery calcium in women during midlife: the CARDIA Study. Circulation. 2021; 143:974–87.
Article68. Ziegler AG, Wallner M, Kaiser I, Rossbauer M, Harsunen MH, Lachmann L, et al. Long-term protective effect of lactation on the development of type 2 diabetes in women with recent gestational diabetes mellitus. Diabetes. 2012; 61:3167–71.
Article69. Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 2012; 129:e827–41.70. Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005; 294:2601–10.
Article71. Gunderson EP, Lewis CE, Lin Y, Sorel M, Gross M, Sidney S, et al. Lactation duration and progression to diabetes in women across the childbearing years: the 30-year CARDIA Study. JAMA Intern Med. 2018; 178:328–37.
Article72. Gunderson EP, Lewis CE, Tsai AL, Chiang V, Carnethon M, Quesenberry CP Jr, et al. A 20-year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes. 2007; 56:2990–6.73. Gunderson EP, Jacobs DR Jr, Chiang V, Lewis CE, Feng J, Quesenberry CP Jr, et al. Duration of lactation and incidence of the metabolic syndrome in women of reproductive age according to gestational diabetes mellitus status: a 20-year prospective study in CARDIA (Coronary Artery Risk Development in Young Adults). Diabetes. 2010; 59:495–504.
Article74. Ajmera VH, Terrault NA, VanWagner LB, Sarkar M, Lewis CE, Carr JJ, et al. Longer lactation duration is associated with decreased prevalence of non-alcoholic fatty liver disease in women. J Hepatol. 2019; 70:126–32.
Article75. Appiah D, Lewis CE, Jacobs DR, Shikany JM, Quesenberry CP, Gross M, et al. The association of lactation duration with visceral and pericardial fat volumes in parous women: the CARDIA Study. J Clin Endocrinol Metab. 2021; 106:1821–31.
Article76. Stuebe AM, Schwarz EB, Grewen K, Rich-Edwards JW, Michels KB, Foster EM, et al. Duration of lactation and incidence of maternal hypertension: a longitudinal cohort study. Am J Epidemiol. 2011; 174:1147–58.
Article77. Nguyen B, Gale J, Nassar N, Bauman A, Joshy G, Ding D. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large Australian Cohort Study. J Am Heart Assoc. 2019; 8:e011056.
Article78. Zhang Z, Lai M, Piro AL, Alexeeff SE, Allalou A, Rost HL, et al. Intensive lactation among women with recent gestational diabetes significantly alters the early postpartum circulating lipid profile: the SWIFT study. BMC Med. 2021; 19:241.
Article79. Banerjee RR, Cyphert HA, Walker EM, Chakravarthy H, Peiris H, Gu X, et al. Gestational diabetes mellitus from inactivation of prolactin receptor and MafB in islet β-cells. Diabetes. 2016; 65:2331–41.
Article80. Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010; 16:804–8.
Article81. Retnakaran R, Ye C, Kramer CK, Connelly PW, Hanley AJ, Sermer M, et al. Maternal serum prolactin and prediction of postpartum β-cell function and risk of prediabetes/diabetes. Diabetes Care. 2016; 39:1250–8.
Article82. Bajaj H, Ye C, Hanley AJ, Connelly PW, Sermer M, Zinman B, et al. Prior lactation reduces future diabetic risk through sustained postweaning effects on insulin sensitivity. Am J Physiol Endocrinol Metab. 2017; 312:E215–23.
Article83. Wang T, Lu J, Xu Y, Li M, Sun J, Zhang J, et al. Circulating prolactin associates with diabetes and impaired glucose regulation: a population-based study. Diabetes Care. 2013; 36:1974–80.84. Ruiz-Herrera X, de Los Rios EA, Diaz JM, Lerma-Alvarado RM, Martinez de la Escalera L, Lopez-Barrera F, et al. Prolactin promotes adipose tissue fitness and insulin sensitivity in obese males. Endocrinology. 2017; 158:56–68.
Article85. Lowe WL Jr, Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. 2018; 320:1005–16.
Article86. Coustan DR, Dyer AR, Metzger BE. One-step or 2-step testing for gestational diabetes: which is better? Am J Obstet Gynecol. 2021; 225:634–44.87. Casey B. Gestational diabetes: on broadening the diagnosis. N Engl J Med. 2021; 384:965–6.