Cancer Res Treat.
2022 Jan;54(1):54-64. 10.4143/crt.2020.1247.
Increased Radiosensitivity of Solid Tumors Harboring ATM and BRCA1/2 Mutations
- Affiliations
-
- 1Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 2Division of Medical Oncology, Department of Internal Medicine, Graduate School of Medical Science, Brain Korea 21 Project, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Radiology and Research Institute of Radiological Science, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 4Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2524587
- DOI: http://doi.org/10.4143/crt.2020.1247
Abstract
- Purpose
Preclinical data indicate that response to radiotherapy (RT) depends on DNA damage repair. In this study, we investigated the role of mutations in genes related to DNA damage repair in treatment outcome after RT.
Materials and Methods
Patients with solid tumor who participated in next generation sequencing panel screening using biopsied tumor tissue between October 2013 and February 2019 were reviewed and 97 patients that received RT were included in this study. Best response to RT and the cumulative local recurrence rate (LRR) were compared according to absence or presence of missense, nonsense, and frameshift mutations in ATM and/or BRCA1/2.
Results
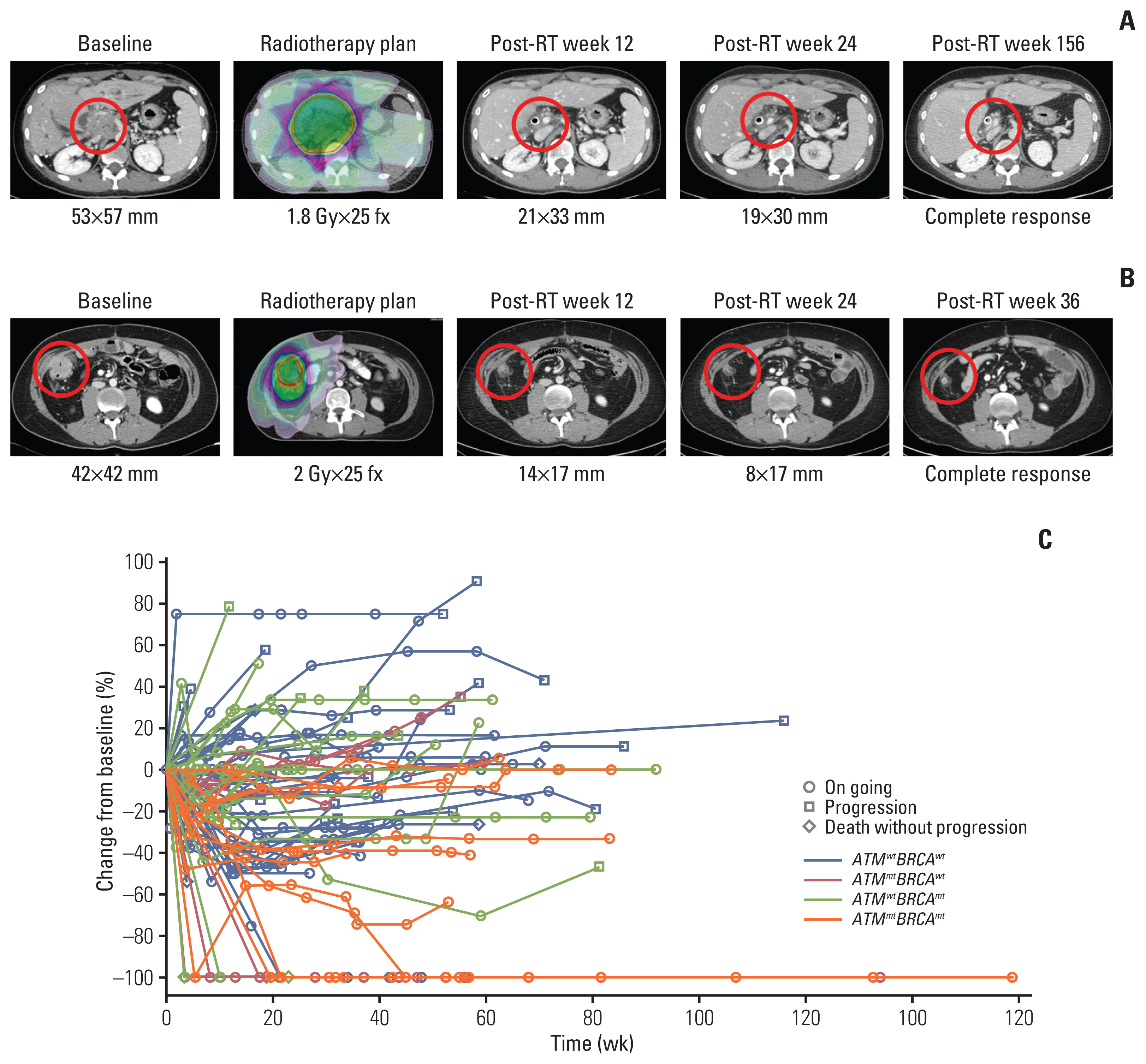
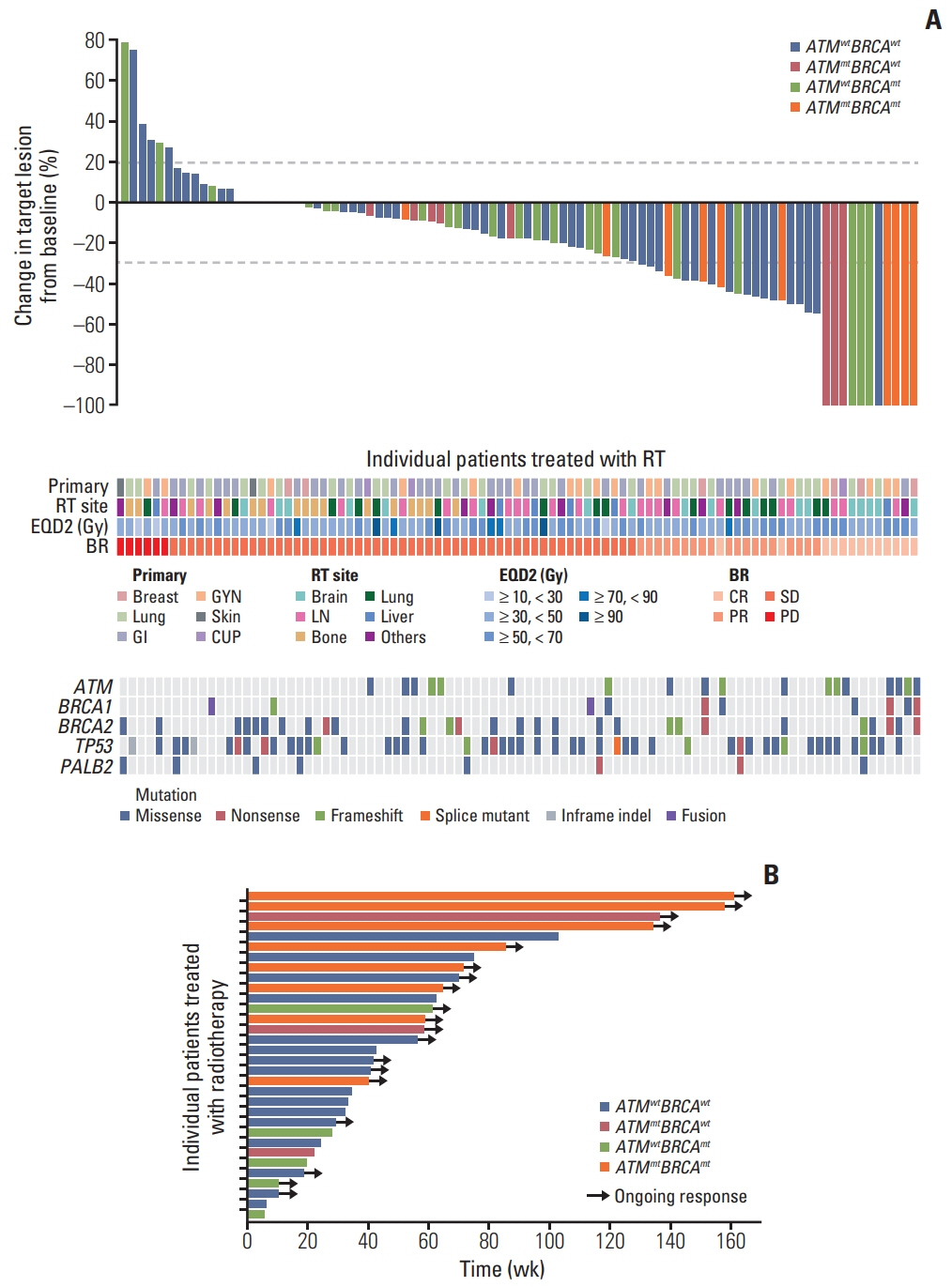
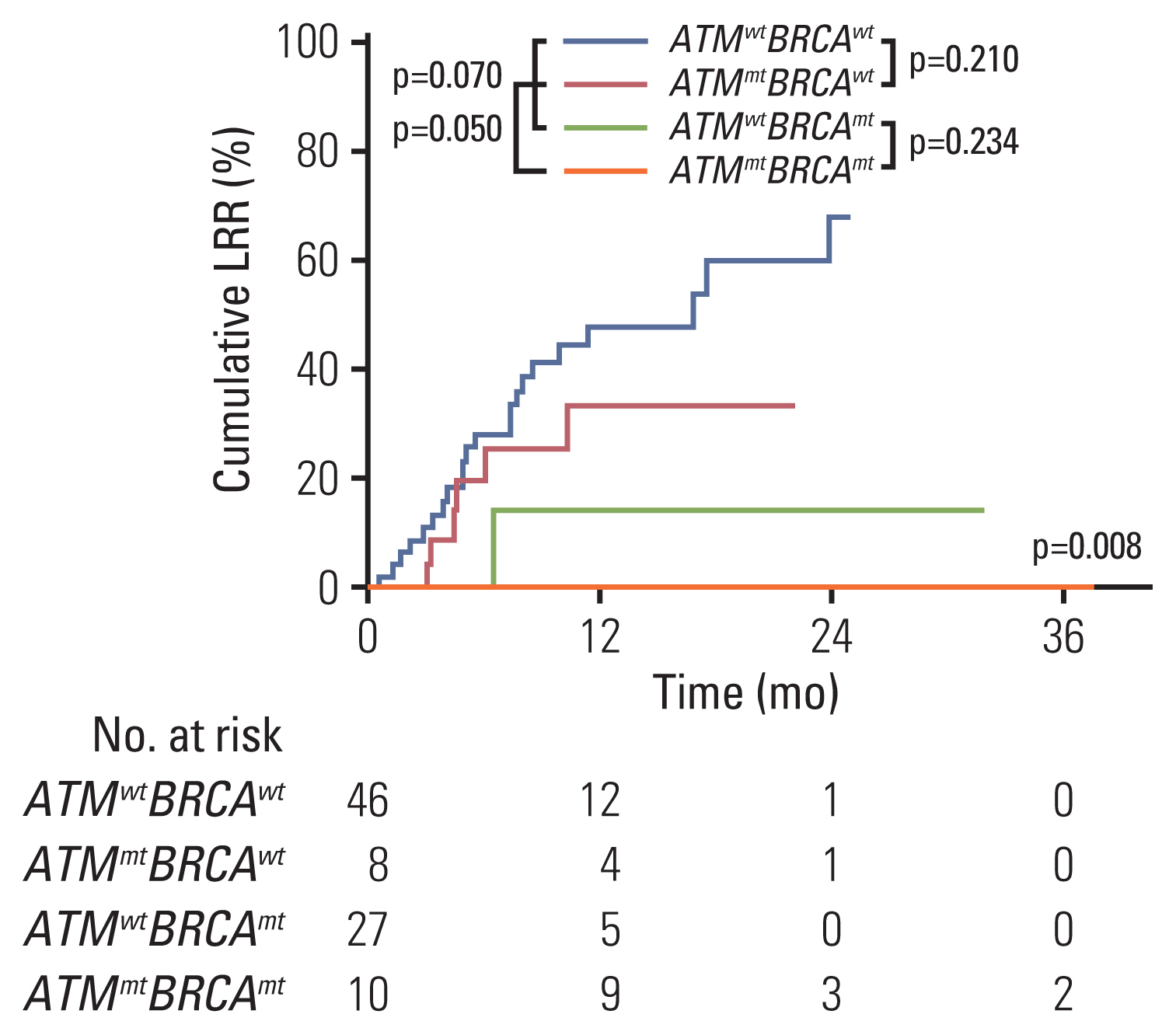
Of the 97 patients, five patients harbored mutation only in ATM, 22 in only BRCA1/2, and six in both ATM and BRCA1/2 (ATMmtBRCAmt). Propensity score matching was performed to select the control group without mutations (ATMwtBRCAwt, n=33). In total, 90 RT-treated target lesions were evaluated in 66 patients. Highest objective response rate of 80% was observed in ATMmtBRCAmt lesions (p=0.007), which was mostly durable. Furthermore, the cumulative 1-year LRR was the lowest in ATMmtBRCAmt lesions and the highest in ATMwtBRCAwt lesions (0% vs. 47.9%, p=0.008). RT-associated toxicities were observed in 10 treatments with no significant difference among the subgroups (p=0.680).
Conclusion
Tumors with ATM and BRCA1/2 mutations exhibited superior tumor response and local control after RT compared to tumors without these mutations. The results are hypothesis generating and suggest the need for integrating the tumor mutation profile of DNA repair genes during treatment planning.
Keyword
Figure
Reference
-
References
1. Barton MB, Jacob S, Shafiq J, Wong K, Thompson SR, Hanna TP, et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014; 112:140–4.
Article2. Round CE, Williams MV, Mee T, Kirkby NF, Cooper T, Hoskin P, et al. Radiotherapy demand and activity in England 2006–2020. Clin Oncol (R Coll Radiol). 2013; 25:522–30.
Article3. Maranzano E, Bellavita R, Rossi R, De Angelis V, Frattegiani A, Bagnoli R, et al. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. J Clin Oncol. 2005; 23:3358–65.
Article4. Klement RJ, Guckenberger M, Alheid H, Allgauer M, Becker G, Blanck O, et al. Stereotactic body radiotherapy for oligo-metastatic liver disease: influence of pre-treatment chemotherapy and histology on local tumor control. Radiother Oncol. 2017; 123:227–33.5. Rades D, Fehlauer F, Schulte R, Veninga T, Stalpers LJ, Basic H, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006; 24:3388–93.
Article6. Gerweck LE, Vijayappa S, Kurimasa A, Ogawa K, Chen DJ. Tumor cell radiosensitivity is a major determinant of tumor response to radiation. Cancer Res. 2006; 66:8352–5.
Article7. Pavlopoulou A, Bagos PG, Koutsandrea V, Georgakilas AG. Molecular determinants of radiosensitivity in normal and tumor tissue: a bioinformatic approach. Cancer Lett. 2017; 403:37–47.
Article8. Hakem R. DNA-damage repair: the good, the bad, and the ugly. EMBO J. 2008; 27:589–605.
Article9. Pollard JM, Gatti RA. Clinical radiation sensitivity with DNA repair disorders: an overview. Int J Radiat Oncol Biol Phys. 2009; 74:1323–31.
Article10. Pierce LJ, Levin AM, Rebbeck TR, Ben-David MA, Friedman E, Solin LJ, et al. Ten-year multi-institutional results of breast-conserving surgery and radiotherapy in BRCA1/2-associated stage I/II breast cancer. J Clin Oncol. 2006; 24:2437–43.
Article11. Choi M, Kipps T, Kurzrock R. ATM nutations in cancer: therapeutic implications. Mol Cancer Ther. 2016; 15:1781–91.12. Hennessy BT, Timms KM, Carey MS, Gutin A, Meyer LA, Flake DD 2nd, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010; 28:3570–6.
Article13. Ahmed KA, Caudell JJ, El-Haddad G, Berglund AE, Welsh EA, Yue B, et al. Radiosensitivity differences between liver metastases based on primary histology suggest implications for clinical outcomes after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2016; 95:1399–404.
Article14. Helou J, Thibault I, Poon I, Chiang A, Jain S, Soliman H, et al. Stereotactic ablative radiation therapy for pulmonary metastases: histology, dose, and indication matter. Int J Radiat Oncol Biol Phys. 2017; 98:419–27.
Article15. Zeng KL, Sahgal A, Husain ZA, Myrehaug S, Tseng CL, Detsky J, et al. Local control and patterns of failure for “Radioresistant” spinal metastases following stereotactic body radiotherapy compared to a “Radiosensitive” reference. J Neurooncol. 2021; 152:173–82.
Article16. Park E, Shim HS. Detection of targetable genetic alterations in Korean lung cancer patients: a comparison study of single-gene assays and targeted next-generation sequencing. Cancer Res Treat. 2020; 52:543–51.
Article17. Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017; 19:4–23.18. Goodwin PJ, Phillips KA, West DW, Ennis M, Hopper JL, John EM, et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol. 2012; 30:19–26.
Article19. Bernstein JL, Haile RW, Stovall M, Boice JD Jr, Shore RE, Langholz B, et al. Radiation exposure, the ATM Gene, and contralateral breast cancer in the women’s environmental cancer and radiation epidemiology study. J Natl Cancer Inst. 2010; 102:475–83.
Article20. Liauw SL, Connell PP, Weichselbaum RR. New paradigms and future challenges in radiation oncology: an update of biological targets and technology. Sci Transl Med. 2013; 5:173sr2.
Article21. Ceccaldi R, Rondinelli B, D’Andrea AD. Repair pathway choices and consequences at the double-strand break. Trends Cell Biol. 2016; 26:52–64.
Article22. Roy R, Chun J, Powell SN. BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011; 12:68–78.
Article23. Shiloh Y. The ATM-mediated DNA-damage response: taking shape. Trends Biochem Sci. 2006; 31:402–10.
Article24. Prakash R, Zhang Y, Feng W, Jasin M. Homologous recombination and human health: the roles of BRCA1, BRCA2, and associated proteins. Cold Spring Harb Perspect Biol. 2015; 7:a016600.
Article25. Marechal A, Zou L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol. 2013; 5:a012716.
Article26. Rainey MD, Charlton ME, Stanton RV, Kastan MB. Transient inhibition of ATM kinase is sufficient to enhance cellular sensitivity to ionizing radiation. Cancer Res. 2008; 68:7466–74.
Article27. Ma J, Setton J, Morris L, Albornoz PB, Barker C, Lok BH, et al. Genomic analysis of exceptional responders to radiotherapy reveals somatic mutations in ATM. Oncotarget. 2017; 8:10312–23.
Article28. Lavin MF, Delia D, Chessa L. ATM and the DNA damage response. Workshop on ataxia-telangiectasia and related syndromes. EMBO Rep. 2006; 7:154–60.29. Lopez-Crapez E, Bibeau F, Thezenas S, Ychou M, Simony-Lafontaine J, Thirion A, et al. p53 status and response to radiotherapy in rectal cancer: a prospective multilevel analysis. Br J Cancer. 2005; 92:2114–21.
Article30. Knijnenburg TA, Wang L, Zimmermann MT, Chambwe N, Gao GF, Cherniack AD, et al. Genomic and molecular landscape of DNA damage repair deficiency across The Cancer Genome Atlas. Cell Rep. 2018; 23:239–54.31. Kuhne M, Riballo E, Rief N, Rothkamm K, Jeggo PA, Lobrich M. A double-strand break repair defect in ATM-deficient cells contributes to radiosensitivity. Cancer Res. 2004; 64:500–8.
Article32. Metcalfe K, Lynch HT, Ghadirian P, Tung N, Olivotto I, Warner E, et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2004; 22:2328–35.
Article33. Zhou B, Latouche A, Rocha V, Fine J. Competing risks regression for stratified data. Biometrics. 2011; 67:661–70.
Article34. Scott JG, Berglund A, Schell MJ, Mihaylov I, Fulp WJ, Yue B, et al. A genome-based model for adjusting radiotherapy dose (GARD): a retrospective, cohort-based study. Lancet Oncol. 2017; 18:202–11.
Article35. Bibault JE, Tinhofer I. The role of next-generation sequencing in tumoral radiosensitivity prediction. Clin Transl Radiat Oncol. 2017; 3:16–20.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of DNA Damage Response Proteins and Associations with Clinicopathologic Characteristics in Chinese Familial Breast Cancer Patients with BRCA1/2 Mutations
- A Study on BRCA1/2 Mutations, Hormone Status and HER-2 Status in Korean Women with Early-onset Breast Cancer
- Novel Germline Mutations of BRCA1 and BRCA2 in Korean Familial Breast Cancer Patients
- Distribution of BRCA1 and BRCA2 Mutations in Asian Patients with Breast Cancer
- Impact of Early Testing and Analysis of Germline Genetic Mutation in Patients with Breast Cancer: A Single Institution Experience