J Pathol Transl Med.
2022 Jan;56(1):22-31. 10.4132/jptm.2021.08.31.
Association of PTTG1 expression with invasiveness of non-functioning pituitary adenomas
- Affiliations
-
- 1Department of Pathology, Keimyung University Dongsan Medical Center, Daegu, Korea
- KMID: 2524226
- DOI: http://doi.org/10.4132/jptm.2021.08.31
Abstract
- Background
Pituitary tumor transforming gene 1 (PTTG1), paired-like homeodomain 2 (PITX2), and galectin-3 have been widely studied as predictive biomarkers for various tumors and are involved in tumorigenesis and tumor progression. We evaluated the usefulness of PTTG1, PITX2, and galectin-3 as predictive biomarkers for invasive non-functioning pituitary adenomas (NFPAs) by determining the relationship between the expressions of these three proteins and the invasiveness of the NFPAs. We also investigated whether PTTG1, E-cadherin, and Ki-67, which are known to be related to each other, show a correlation with NFPA features.
Methods
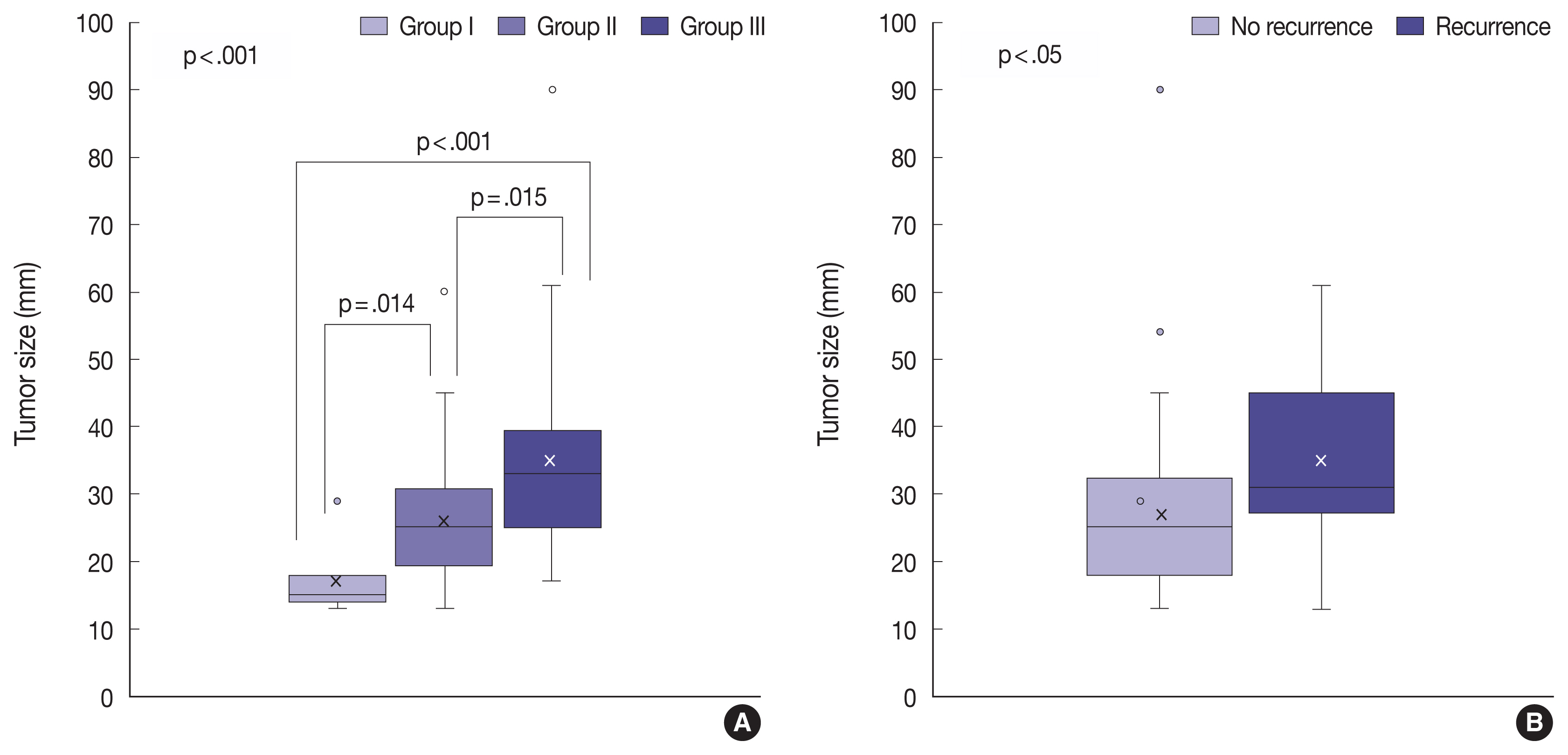
A retrospective study was conducted on 87 patients with NPFAs who underwent surgical removal. The NFPAs were classified into three groups based on magnetic resonance imaging findings of suprasellar extension and cavernous sinus invasion. Immunohistochemical staining for PTTG1, PITX2, galectin-3, E-cadherin, and Ki-67 was performed on tissue microarrays.
Results
PTTG1 expression showed a statistically significant correlation with the invasiveness of NFPAs, whereas PITX2 and galectin-3 did not have a relationship with the invasiveness of NFPAs. Moreover, there was no association among PTTG1, E-cadherin, and Ki-67 expression.
Conclusions
PTTG1 has the potential to serve as a predictive biomarker for invasive NFPA. Furthermore, this study may serve as a reference for the development of PTTG1-targeted therapeutic agents.
Keyword
Figure
Reference
-
References
1. Gadelha MR, Trivellin G, Hernandez Ramirez LC, Korbonits M. Genetics of pituitary adenomas. Front Horm Res. 2013; 41:111–40.
Article2. Greenman Y, Cooper O, Yaish I, et al. Treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists. Eur J Endocrinol. 2016; 175:63–72.
Article3. Greenman Y, Tordjman K, Osher E, et al. Postoperative treatment of clinically nonfunctioning pituitary adenomas with dopamine agonists decreases tumour remnant growth. Clin Endocrinol (Oxf). 2005; 63:39–44.
Article4. DeLellis RA, Lloyd RV, Heitz PU, Eng C. World Health Organization classification of tumours: pathology and genetics of tumours of endocrine organs. Lyons: IARC Press;2004.5. Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO cassification of tumours of endocrine organs. Lyons: IARC Press;2012. p. 11–3.6. Saeger W, Ludecke DK, Buchfelder M, Fahlbusch R, Quabbe HJ, Petersenn S. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur J Endocrinol. 2007; 156:203–16.
Article7. Zada G, Woodmansee WW, Ramkissoon S, Amadio J, Nose V, Laws ER Jr. Atypical pituitary adenomas: incidence, clinical characteristics, and implications. J Neurosurg. 2011; 114:336–44.
Article8. Scoazec JY, Couvelard A, Reseau T. Classification of pancreatic neuroendocrine tumours: changes made in the 2017 WHO classification of tumours of endocrine organs and perspectives for the future. Ann Pathol. 2017; 37:444–56.9. Trouillas J, Roy P, Sturm N, et al. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case-control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 2013; 126:123–35.10. Matsuyama J. Ki-67 expression for predicting progression of postoperative residual pituitary adenomas: correlations with clinical variables. Neurol Med Chir (Tokyo). 2012; 52:563–9.
Article11. Chiloiro S, Doglietto F, Trapasso B, et al. Typical and atypical pituitary adenomas: a single-center analysis of outcome and prognosis. Neuroendocrinology. 2015; 101:143–50.
Article12. Kim JS, Lee YS, Jung MJ, Hong YK. The predictive value of pathologic features in pituitary adenoma and correlation with pituitary adenoma recurrence. J Pathol Transl Med. 2016; 50:419–25.
Article13. Mooney MA, Hardesty DA, Sheehy JP, et al. Rater reliability of the hardy classification for pituitary adenomas in the magnetic resonance imaging era. J Neurol Surg B Skull Base. 2017; 78:413–8.
Article14. Thapar K, Scheithauer BW, Kovacs K, Pernicone PJ, Laws ER Jr. p53 expression in pituitary adenomas and carcinomas: correlation with invasiveness and tumor growth fractions. Neurosurgery. 1996; 38:765–70.
Article15. Oliveira MC, Marroni CP, Pizarro CB, Pereira-Lima JF, Barbosa-Coutinho LM, Ferreira NP. Expression of p53 protein in pituitary adenomas. Braz J Med Biol Res. 2002; 35:561–5.
Article16. Salehi F, Agur A, Scheithauer BW, Kovacs K, Lloyd RV, Cusimano M. Biomarkers of pituitary neoplasms: a review (Part II). Neurosurgery. 2010; 67:1790–8.
Article17. Sav A, Rotondo F, Syro LV, Scheithauer BW, Kovacs K. Biomarkers of pituitary neoplasms. Anticancer Res. 2012; 32:4639–54.18. McCabe CJ, Khaira JS, Boelaert K, et al. Expression of pituitary tumour transforming gene (PTTG) and fibroblast growth factor-2 (FGF-2) in human pituitary adenomas: relationships to clinical tumour behaviour. Clin Endocrinol (Oxf). 2003; 58:141–50.
Article19. Shah PP, Kakar SS. Pituitary tumor transforming gene induces epithelial to mesenchymal transition by regulation of Twist, Snail, Slug, and E-cadherin. Cancer Lett. 2011; 311:66–76.
Article20. Zou H, McGarry TJ, Bernal T, Kirschner MW. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science. 1999; 285:418–22.
Article21. Salehi F, Kovacs K, Scheithauer BW, Lloyd RV, Cusimano M. Pituitary tumor-transforming gene in endocrine and other neoplasms: a review and update. Endocr Relat Cancer. 2008; 15:721–43.
Article22. Feng W, Xiaoyan X, Shenglei L, Hongtao L, Guozhong J. PTTG1 cooperated with GLI1 leads to epithelial-mesenchymal transition in esophageal squamous cell cancer. Oncotarget. 2017; 8:92388–400.
Article23. Yoon CH, Kim MJ, Lee H, et al. PTTG1 oncogene promotes tumor malignancy via epithelial to mesenchymal transition and expansion of cancer stem cell population. J Biol Chem. 2012; 287:19516–27.
Article24. Nangia-Makker P, Honjo Y, Sarvis R, et al. Galectin-3 induces endothelial cell morphogenesis and angiogenesis. Am J Pathol. 2000; 156:899–909.
Article25. Yoshii T, Fukumori T, Honjo Y, Inohara H, Kim HR, Raz A. Galectin-3 phosphorylation is required for its anti-apoptotic function and cell cycle arrest. J Biol Chem. 2002; 277:6852–7.
Article26. Cottier JP, Destrieux C, Brunereau L, et al. Cavernous sinus invasion by pituitary adenoma: MR imaging. Radiology. 2000; 215:463–9.
Article27. Zhang X, Horwitz GA, Heaney AP, et al. Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J Clin Endocrinol Metab. 1999; 84:761–7.
Article28. Raverot G, Wierinckx A, Dantony E, et al. Prognostic factors in prolactin pituitary tumors: clinical, histological, and molecular data from a series of 94 patients with a long postoperative follow-up. J Clin Endocrinol Metab. 2010; 95:1708–16.
Article29. Li Y, Zhou LP, Ma P, et al. Relationship of PTTG expression with tumor invasiveness and microvessel density of pituitary adenomas: a meta-analysis. Genet Test Mol Biomarkers. 2014; 18:279–85.
Article30. Trott G, Ongaratti BR, de Oliveira Silva CB, et al. PTTG overexpression in non-functioning pituitary adenomas: Correlation with invasiveness, female gender and younger age. Ann Diagn Pathol. 2019; 41:83–9.
Article31. Filippella M, Galland F, Kujas M, et al. Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: a clinical and immunohistochemical study. Clin Endocrinol (Oxf). 2006; 65:536–43.
Article32. Noh TW, Jeong HJ, Lee MK, Kim TS, Kim SH, Lee EJ. Predicting recurrence of nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2009; 94:4406–13.
Article33. Tamura R, Ohara K, Morimoto Y, et al. PITX2 expression in nonfunctional pituitary neuroendocrine tumor with cavernous sinus invasion. Endocr Pathol. 2019; 30:81–9.
Article34. Acunzo J, Roche C, Defilles C, et al. Inactivation of PITX2 transcription factor induced apoptosis of gonadotroph tumoral cells. Endocrinology. 2011; 152:3884–92.
Article35. Jin L, Riss D, Ruebel K, et al. Galectin-3 expression in functioning and silent ACTH-producing adenomas. Endocr Pathol. 2005; 16:107–14.
Article36. Zhang Y, He N, Zhou J, Chen Y. The relationship between MRI invasive features and expression of EMMPRIN, galectin-3, and microvessel density in pituitary adenoma. Clin Imaging. 2011; 35:165–73.
Article37. Righi A, Morandi L, Leonardi E, et al. Galectin-3 expression in pituitary adenomas as a marker of aggressive behavior. Hum Pathol. 2013; 44:2400–9.
Article38. Qian ZR, Sano T, Yoshimoto K, et al. Tumor-specific downregulation and methylation of the CDH13 (H-cadherin) and CDH1 (Ecadherin) genes correlate with aggressiveness of human pituitary adenomas. Mod Pathol. 2007; 20:1269–77.
Article39. Wong TS, Gao W, Chan JY. Interactions between E-cadherin and microRNA deregulation in head and neck cancers: the potential interplay. Biomed Res Int. 2014; 2014:126038.
Article40. Heaney AP, Horwitz GA, Wang Z, Singson R, Melmed S. Early involvement of estrogen-induced pituitary tumor transforming gene and fibroblast growth factor expression in prolactinoma pathogenesis. Nat Med. 1999; 5:1317–21.
Article41. Wang Z, Moro E, Kovacs K, Yu R, Melmed S. Pituitary tumor transforming gene-null male mice exhibit impaired pancreatic beta cell proliferation and diabetes. Proc Natl Acad Sci U S A. 2003; 100:3428–32.
Article42. Mu YM, Oba K, Yanase T, et al. Human pituitary tumor transforming gene (hPTTG) inhibits human lung cancer A549 cell growth through activation of p21(WAF1/CIP1). Endocr J. 2003; 50:771–81.43. Yu R, Cruz-Soto M, Li Calzi S, Hui H, Melmed S. Murine pituitary tumor-transforming gene functions as a securin protein in insulin-secreting cells. J Endocrinol. 2006; 191:45–53.
Article44. Dekkers OM, Karavitaki N, Pereira AM. The epidemiology of aggressive pituitary tumors (and its challenges). Rev Endocr Metab Disord. 2020; 21:209–12.
Article45. Roelfsema F, Biermasz NR, Pereira AM. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary. 2012; 15:71–83.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidemiology of Functioning Pituitary Adenomas
- Expression of Serum PTTG1 in Laryngeal Carcinoma and Its Correlation to Prognosis
- Silent Adenomas of Pituitary Gland: It's Immunohistochemical Features and Clinical Characteristics
- Comparative evaluation of CT and secreting hormones in pituitary adenomas
- Pituitary Adenoma Confined Within the Pituitary Stalk: A Case Report and Literature Review