J Cerebrovasc Endovasc Neurosurg.
2021 Dec;23(4):359-364. 10.7461/jcen.2021.E2021.06.004.
Staged hybrid treatment for giant thrombosed fusiform aneurysm
- Affiliations
-
- 1Department of Neurosurgery, Cheonan Hospital, Soonchunhyang University School of Medicine, Cheonan, Korea
- 2Department of Cardiothoracic surgery, Cheonan Hospital, Soonchunhyang University School of Medicine, Cheonan, Korea
- KMID: 2523895
- DOI: http://doi.org/10.7461/jcen.2021.E2021.06.004
Abstract
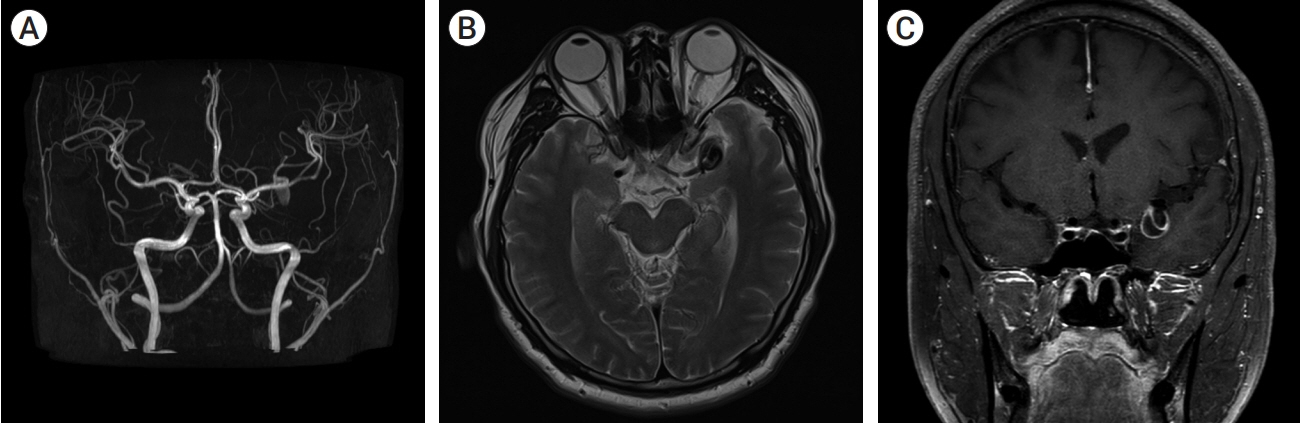
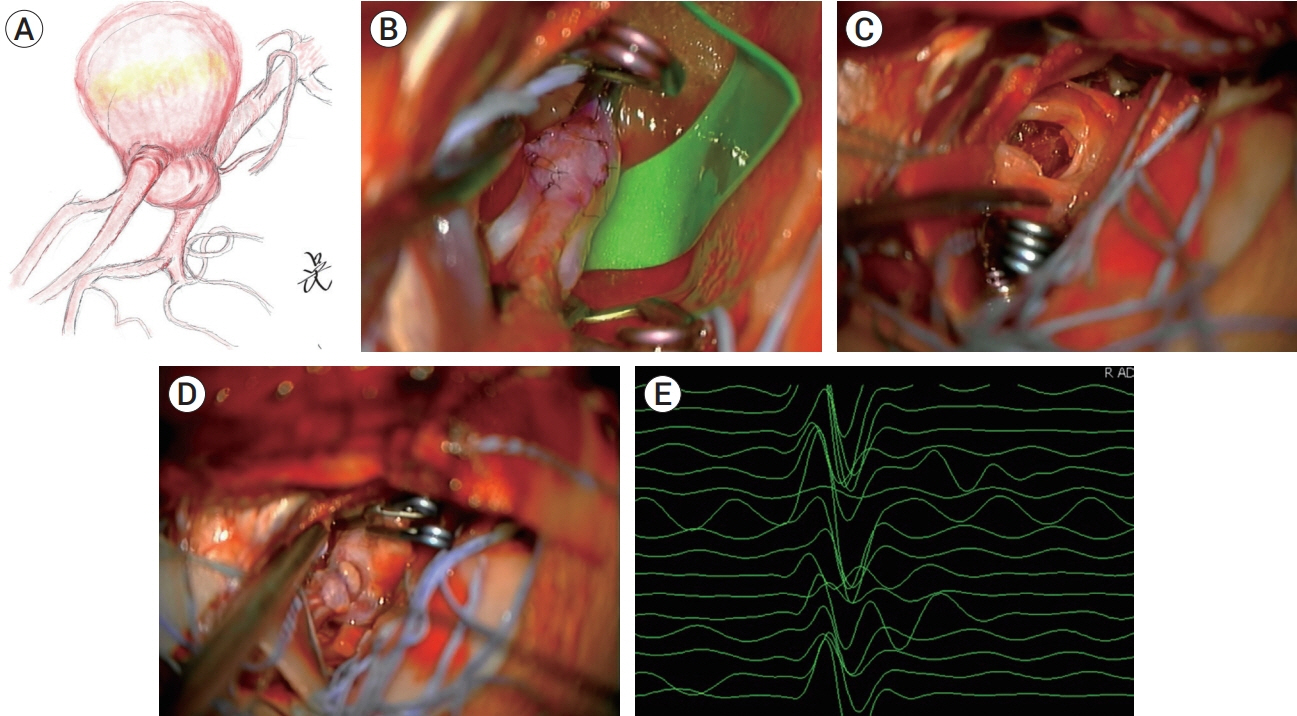
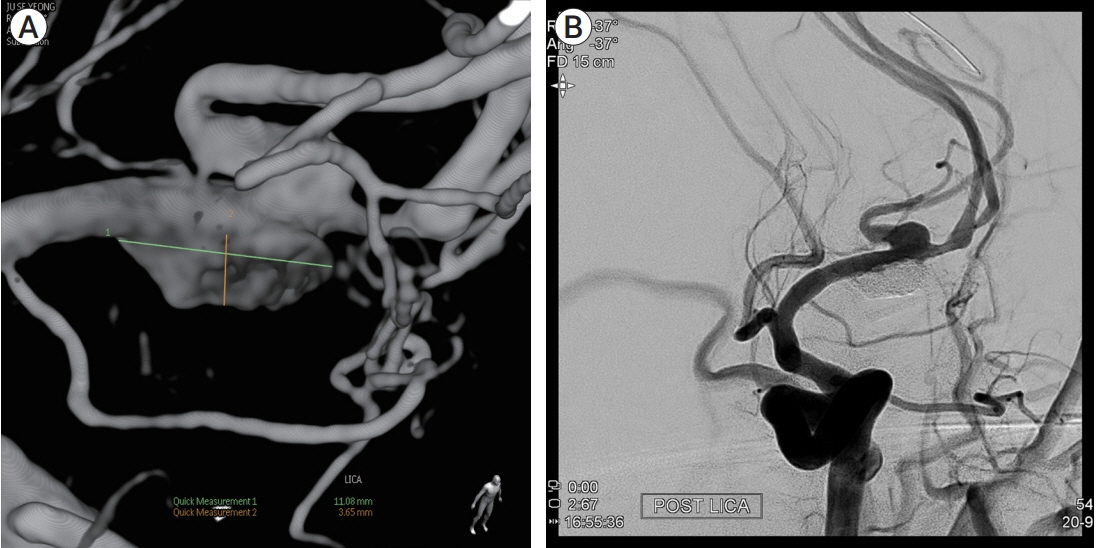
- Partially thrombosed intracranial aneurysm was difficult to treat because of higher recurrence rate compared to non-thrombosed saccular aneurysm. The author reports a case of partially thrombosed intracranial aneurysm causing transient ischemic symptom. A 40-year-old man presented with transient right hemiparesis. Brain magnetic resonance imaging (MRI) depicted low-signal intensity target-like mass lesion on left sylvian fissure, and magnetic resonance angiography (MRA) showed aneurysm on left middle cerebral artery bifurcation (MCBF), suggested thrombosed aneurysm. On operative finding, aneurysm wall had thick and atherosclerotic change, and it was fusiform aneurysm not saccular type. We initially planned direct clip for the aneurysm, but it was failed due to collapse of parent artery after clipping on aneurysm neck. To prevent ischemia, extracranial-intracranial bypass was performed and then thrombectomy with clip reconstruction. To remodeling the fusiform aneurysm, stent-assisted coiling was performed for remnant portion of aneurysm. With staged hybrid technique, giant thrombosed fusiform aneurysm was completely obliterated and the patient did not suffer any neurologic symptoms no longer.
Keyword
Figure
Reference
-
1. Chalouhi N, Tjoumakaris SI, Gonzalez LF, Hasan D, Pema PJ, Gould G, et al. Spontaneous delayed migration/shortening of the pipeline embolization device: report of 5 cases. American Journal of Neuroradiology. 2013; Dec. 34(12):2326–30.
Article2. Darsaut TE, Bing F, Salazkin I, Gevry G, Raymond J. Flow diverters failing to occlude experimental bifurcation or curved sidewall aneurysms: an in vivo study in canines. J Neurosurg. 2012; Jul. 117(1):37–44.
Article3. Ferns SP, van Rooij WJ, Sluzewski M, van den Berg R, Majoie CBLM. Partially thrombosed intracranial aneurysms presenting with mass effect: long-term clinical and imaging follow-up after endovascular treatment. American Journal of Neuroradiology. 2010; Aug. 31(7):1197–205.
Article4. Ferns SP, Sprengers MES, van Rooij WJ, Rinkel GJE, van Rijn JC, Bipat S, et al. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke. 2009; Aug. 40(8):e523–9.5. Güresir E, Wispel C, Borger V, Hadjiathanasiou A, Vatter H, Schuss P. Treatment of partially thrombosed intracranial aneurysms: single-center series and systematic review. World Neurosurgery. 2018; Oct. 118:e834–41.
Article6. Iihara K, Murao K, Sakai N, Soeda A, Ishibashi-Ueda H, Yutani C, et al. Continued growth of and increased symptoms from a thrombosed giant aneurysm of the vertebral artery after complete endovascular occlusion and trapping: the role of vasa vasorum: case report. J Neurosurg. 2003; Feb. 98(2):407–13.7. Iihara K, Murao K, Yamada N, Takahashi JC, Nakajima N, Satow T, et al. Growth potential and response to multimodality treatment of partially thrombosed large or giant aneurysms in the posterior circulation. Neurosurgery. 2008; Nov. 63(5):832–44.
Article8. Kim HJ, Lee SW, Lee TH, Kim YS. Huge intramural hematoma in a thrombosed middle cerebral artery aneurysm: a case report. Journal of Cerebrovascular and Endovascular Neurosurgery. 2015; 17(3):234–8.
Article9. Kim YJ, Ko JH. Endovascular treatment of a large partially thrombosed basilar tip aneurysm. Journal of Korean Neurosurgical Society. 2012; Jan. 51(1):62–5.
Article10. Krings T, Lasjaunias P, Geibprasert S, Pereira V, Hans FJ. The aneurysmal wall. The key to a subclassification of intracranial arterial aneurysm vasculopathies? Interventional Neuroradiology. 2008; Sep. 14(1_suppl):39–47.11. Krings T, Piske RL, Lasjaunias PL. Intracranial arterial aneurysm vasculopathies: targeting the outer vessel wall. Neurosurgery. 2005; Dec. 47(12):931–7.
Article12. Lawton MT, Quiñones-Hinojosa A, Chang EF, Yu T. Thrombotic intracranial aneurysms: classification scheme and management strategies in 68 patients. Neurosurgery. 2005; Mar. 56(3):441–54.
Article13. Nagahiro S, Takada A, Goto S, Kai Y, Ushio Y. Thrombosed growing giant aneurysms of the vertebral artery: growth mechanism and management. J Neurosurg. 1995; May. 82(5):796–801.
Article14. Scerrati A, Sabatino G, Della Pepa GM, Albanese A, Marchese E, Puca A, et al. Treatment and outcome of thrombosed aneurysms of the middle cerebral artery: institutional experience and a systematic review. Neurosurgical Review. 2019; Sep. 42(3):649–61.
Article15. Sughrue ME, Saloner D, Rayz VL, Lawton MT. Giant intracranial aneurysms: evolution of management in a contemporary surgical series. Neurosurgery. 2011; Dec. 69(6):1261–70.
Article16. Szikora I, Turányi E, Marosfoi M. Evolution of flow-diverter endothelialization and thrombus organization in giant fusiform aneurysms after flow diversion: a histopathologic study. American Journal of Neuroradiology. 2015; Sep. 36(9):1716–20.
Article17. Yang K, Park JC, Ahn JS, Kwon DH, Kwun BD, Kim CJ. Characteristics and outcomes of varied treatment modalities for partially thrombosed intracranial aneurysms: a review of 35 cases. Acta Neurochirurgica. 2014; Sep. 156(9):1669–75.
Article18. Yasui T, Sakamoto H, Kishi H, Komiyama M, Iwai Y, Yamanaka K, et al. Rupture mechanism of a thrombosed slow-growing giant aneurysm of the vertebral artery - case report. Neurologia Medico-Chirurgica. 1998; Dec. 38(12):860–4.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Giant Serpentine Intracranial Aneurysm Treated with Wrapping under the Extracorporeal Circulation and Hypothermia
- A Giant Fusiform Aneurysm of Posterior Cerebral Artery Treated with Trapping after Temporal Lobectomy
- Aneurysmectomy and graft interposition for giant thrombosed proximal internal carotid artery aneurysm: Technical details
- A Case Report of Giant Posterior Inferior Cerebellar Artery Aneurysm Simulating a Posterior Fossa Tumor
- Recanalization of Totally Thrombosed Giant Aneurysm: Case Report