Ann Pediatr Endocrinol Metab.
2021 Dec;26(4):278-283. 10.6065/apem.2142010.005.
Pasireotide treatment for severe congenital hyperinsulinism due to a homozygous ABCC8 mutation
- Affiliations
-
- 1Department of Pediatric Endocrinology, Emma Children’s Hospital, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
- 2Department of Pediatric Endocrinology, Beatrix Children’s Hospital, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands
- 3Department of Pediatric Surgery, Evangelisches Klinikum Bethel, Bielefeld, Germany
- 4Department of Clinical Genetics, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
- 5Department of Pediatrics, Ottovon-Guericke University, Magdeburg, Germany
- 6Department of Pediatric Surgery, Emma Children’s Hospital, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
- KMID: 2523835
- DOI: http://doi.org/10.6065/apem.2142010.005
Abstract
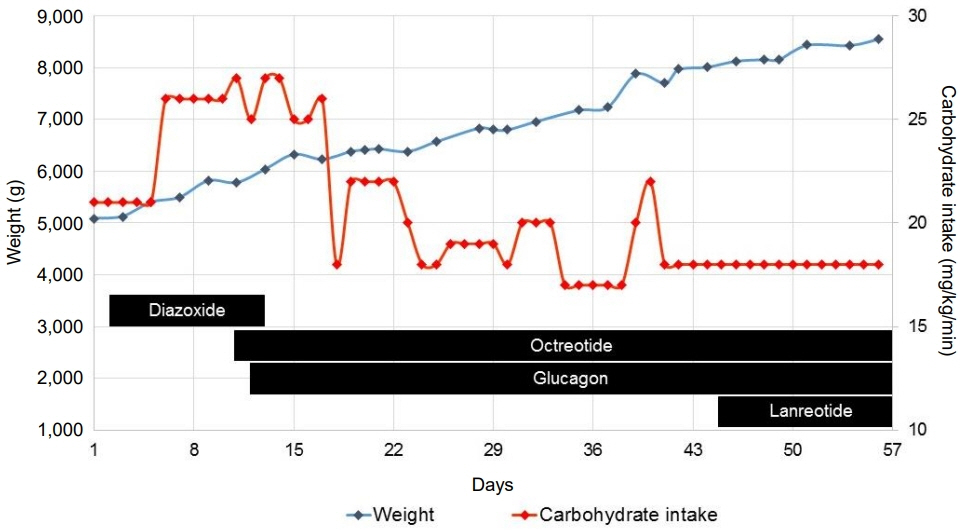
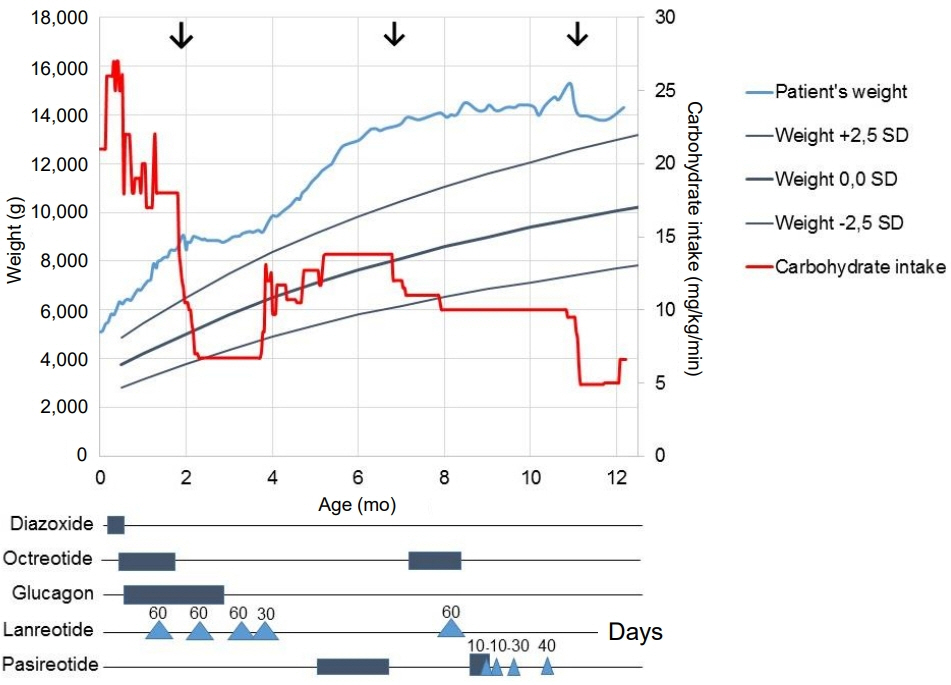
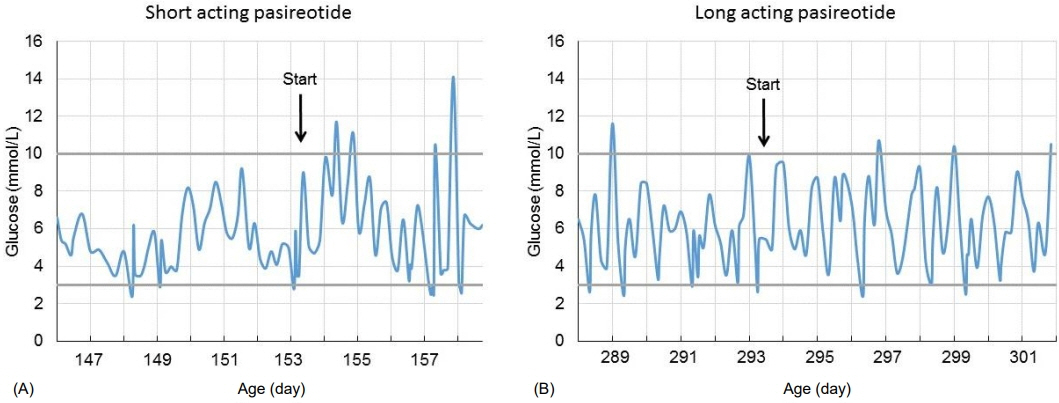
- ABCC8 and KCJN11 mutations cause the most severe diazoxide-resistant forms of congenital hyperinsulinism (CHI). Somatostatin analogues are considered as secondline treatment in diazoxide-unresponsive cases. Current treatment protocols include the first-generation somatostatin analogue octreotide, although pasireotide, a second-generation somatostatin analogue, might be more effective in reducing insulin secretion. Herein we report the first off-label use of pasireotide in a boy with a severe therapy-resistant form of CHI due to a homozygous ABCC8 mutation. After partial pancreatectomy, hyperinsulinism persisted; in an attempt to prevent further surgery, off-label treatment with pasireotide was initiated. Short-acting pasireotide treatment caused high blood glucose level shortly after injection. Long-acting pasireotide treatment resulted in more stable glycemic control. No side effects (e.g., central adrenal insufficiency) were noticed during a 2-month treatment period. Because of recurrent hypoglycemia despite a rather high carbohydrate intake, the boy underwent near-total pancreatectomy at the age of 11 months. In conclusion, pasireotide treatment slightly improved glycemic control without side effects in a boy with severe CHI. However, the effect of pasireotide was not sufficient to prevent near-total pancreatectomy in this case of severe CHI.
Figure
Reference
-
References
1. Gϋemes M, Rahman SA, Kapoor RR, Flanagan S, Houghton JAL, Misra S, et al. Hyperinsulinemic hypoglycemia in children and adolescents: recent advances in understanding of pathophysiology and management. Rev Endocr Metab Disord. 2020; 21:577–97.
Article2. Galcheva S, Al-Khawaga S, Hussain K. Diagnosis and management of hyperinsulinaemic hypoglycaemia. Best Pract Res Clin Endocrinol Metab. 2018; 32:551–73.
Article3. Demirbilek H, Hussain K. Congenital hyperinsulinism: diagnosis and treatment update. J Clin Res Pediatr Endocrinol. 2017; 9(Suppl 2):69–87.
Article4. De Cosio AP, Thornton P. Current and emerging agents for the treatment of hypoglycemia in patients with congenital hyperinsulinism. Paediatr Drugs. 2019; 21:123–36.
Article5. Banerjee I, Salomon-Estebanez M, Shah P, Nicholson J, Cosgrove KE, Dunne MJ. Therapies and outcomes of congenital hyperinsulinism-induced hypoglycaemia. Diabet Med. 2019; 36:9–21.
Article6. Modan-Moses D, Koren I, Mazor-Aronovitch K, Pinhas-Hamiel O, Landau H. Treatment of congenital hyperinsulinism with lanreotide acetate (Somatuline Autogel). J Clin Endocrinol Metab. 2011; 96:2312–7.
Article7. Shah P, Rahman SA, McElroy S, Gilbert C, Morgan K, Hinchey L, et al. Use of long-acting somatostatin analogue (lanreotide) in an adolescent with diazoxide-responsive congenital hyperinsulinism and its psychological impact. Horm Res Paediatr. 2015; 84:355–60.
Article8. van der Steen I, van Albada ME, Mohnike K, Christesen HT, Empting S, Salomon-Estebanez M, et al. A Multicenter experience with long-acting somatostatin analogues in patients with congenital hyperinsulinism. Horm Res Paediatr. 2018; 89:82–9.
Article9. Gomes-Porras M, Cárdenas-Salas J, Álvarez-Escolá C. Somatostatin analogs in clinical practice: a review. Int J Mol Sci. 2020; 21:1682.
Article10. de Heide LJ, Laskewitz AJ, Apers JA. Treatment of severe postRYGB hyperinsulinemic hypoglycemia with pasireotide: a comparison with octreotide on insulin, glucagon, and GLP-1. Surg Obes Relat Dis. 2014; 10:e31–3.
Article11. Schwetz V, Horvath K, Kump P, Lackner C, Perren A, Forrer F, et al. Successful medical treatment of adult nesidioblastosis with pasireotide over 3 years: a case report. Medicine (Baltimore). 2016; 95:e3272.12. Jindal R, Ahmad A, Siddiqui MA, Kochar IS, Wangnoo SK. Novel mutation c.597_598dup in exon 5 of ABCC8 gene causing congenital hyperinsulinism. Diabetes Metab Syndr. 2014; 8:45–7.
Article13. Beltrand J, Caquard M, Arnoux JB, Laborde K, Velho G, Verkarre V, et al. Glucose metabolism in 105 children and adolescents after pancreatectomy for congenital hyperinsulinism. Diabetes Care. 2012; 35:198–203.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A novel mutation of ABCC8 gene in a patient with diazoxide-unresponsive congenital hyperinsulinism
- Congenital hyperinsulinism: current status and future perspectives
- Congenital hyperinsulinism: 2 case reports with different rare variants in ABCC8
- Using low-dose octreotide with diazoxide-resistant congenital hyperinsulinism resulting from compound heterozygous mutations in the ABCC8 gene
- A Case of 2-Month-Old Infant with Persistent Hyperinsulinemic Hypoglycemia Presenting as Atonic Seizure