Ann Pediatr Endocrinol Metab.
2014 Jun;19(2):57-68. 10.6065/apem.2014.19.2.57.
Congenital hyperinsulinism: current status and future perspectives
- Affiliations
-
- 1Department of Pediatric Endocrinology and Metabolism, Children's Medical Center, Osaka City General Hospital, Osaka, Japan. t-yorifuji@hospital.city.osaka.jp
- KMID: 1803855
- DOI: http://doi.org/10.6065/apem.2014.19.2.57
Abstract
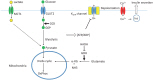
- The diagnosis and treatment of congenital hyperinsulinism (CHI) have made a remarkable progress over the past 20 years and, currently, it is relatively rare to see patients who are left with severe psychomotor delay. The improvement was made possible by the recent developments in the understanding of the molecular and pathological basis of CHI. Known etiologies include inactivating mutations of the K(ATP) channel genes (ABCC8 and KCNJ11) and HNF4A, HNF1A, HADH, and UCP2 or activating mutations of GLUD1, GCK, and SLC16A1. The understanding of the focal form of K(ATP) channel CHI and its detection by 18F-fluoro-L-DOPA positron emission tomography have revolutionized the management of CHI, and many patients can be cured without postoperative diabetes mellitus. The incidence of the focal form appears to be higher in Asian countries; therefore, the establishment of treatment systems is even more important in this population. In addition to diazoxide or long-term subcutaneous infusion of octreotide or glucagon, long-acting octreotide or lanreotide have also been used successfully until spontaneous remission. Because of these medications, near-total pancreatectomy is less often performed even for the diazoxide-unresponsive diffuse form of CHI. Other promising medications include pasireotide, small-molecule correctors such as sulfonylurea or carbamazepine, GLP1 receptor antagonists, or mammalian target of rapamycin inhibitors. Unsolved questions in this field include the identification of the remaining genes responsible for CHI, the mechanisms leading to transient CHI, and the mechanisms responsible for the spontaneous remission of CHI. This article reviews recent developments and hypothesis regarding these questions.
Keyword
MeSH Terms
-
Asian Continental Ancestry Group
Carbamazepine
Congenital Hyperinsulinism*
Diabetes Mellitus
Diagnosis
Diazoxide
Glucagon
Humans
Hyperinsulinism
Hypoglycemia
Incidence
Infusions, Subcutaneous
Octreotide
Pancreatectomy
Positron-Emission Tomography
Remission, Spontaneous
Sirolimus
Carbamazepine
Diazoxide
Glucagon
Octreotide
Sirolimus
Figure
Cited by 2 articles
-
Congenital hyperinsulinism: diagnostic and management challenges in a developing country – case report
Cheri Mathews John, Prakash Agarwal, Suriyakumar Govindarajulu, Sandhya Sundaram, Senthil Senniappan
Ann Pediatr Endocrinol Metab. 2017;22(4):272-275. doi: 10.6065/apem.2017.22.4.272.Congenital hyperinsulinism: 2 case reports with different rare variants in
ABCC8
Julie Mouron-Hryciuk, Sophie Stoppa-Vaucher, Kanetee Busiah, Thérèse Bouthors, Maria Christina Antoniou, Eric Jacot, Klaus Brusgaard, Henrik Thybo Christesen, Khalid Hussain, Andrew Dwyer, Matthias Roth-Kleiner, Michael Hauschild
Ann Pediatr Endocrinol Metab. 2021;26(1):60-65. doi: 10.6065/apem.2040042.021.
Reference
-
1. Menni F, de Lonlay P, Sevin C, Touati G, Peigne C, Barbier V, et al. Neurologic outcomes of 90 neonates and infants with persistent hyperinsulinemic hypoglycemia. Pediatrics. 2001; 107:476–479. PMID: 11230585.
Article2. Steinkrauss L, Lipman TH, Hendell CD, Gerdes M, Thornton PS, Stanley CA. Effects of hypoglycemia on developmental outcome in children with congenital hyperinsulinism. J Pediatr Nurs. 2005; 20:109–118. PMID: 15815570.
Article3. Ludwig A, Ziegenhorn K, Empting S, Meissner T, Marquard J, Holl R, et al. Glucose metabolism and neurological outcome in congenital hyperinsulinism. Semin Pediatr Surg. 2011; 20:45–49. PMID: 21186004.
Article4. Avatapalle HB, Banerjee I, Shah S, Pryce M, Nicholson J, Rigby L, et al. Abnormal neurodevelopmental outcomes are common in children with transient congenital hyperinsulinism. Front Endocrinol (Lausanne). 2013; 4:60. PMID: 23730298.
Article5. Shilyansky J, Fisher S, Cutz E, Perlman K, Filler RM. Is 95% pancreatectomy the procedure of choice for treatment of persistent hyperinsulinemic hypoglycemia of the neonate? J Pediatr Surg. 1997; 32:342–346. PMID: 9044150.
Article6. Meissner T, Wendel U, Burgard P, Schaetzle S, Mayatepek E. Long-term follow-up of 114 patients with congenital hyperinsulinism. Eur J Endocrinol. 2003; 149:43–51. PMID: 12824865.
Article7. Levy-Shraga Y, Pinhas-Hamiel O, Kraus-Houminer E, Landau H, Mazor-Aronovitch K, Modan-Moses D, et al. Cognitive and developmental outcome of conservatively treated children with congenital hyperinsulinism. J Pediatr Endocrinol Metab. 2013; 26:301–308. PMID: 23327786.
Article8. Hussain K. Diagnosis and management of hyperinsulinaemic hypoglycaemia of infancy. Horm Res. 2008; 69:2–13. PMID: 18059080.
Article9. Arnoux JB, Verkarre V, Saint-Martin C, Montravers F, Brassier A, Valayannopoulos V, et al. Congenital hyperinsulinism: current trends in diagnosis and therapy. Orphanet J Rare Dis. 2011; 6:63. PMID: 21967988.
Article10. Mohamed Z, Arya VB, Hussain K. Hyperinsulinaemic hypoglycaemia:genetic mechanisms, diagnosis and management. J Clin Res Pediatr Endocrinol. 2012; 4:169–181. PMID: 23032149.
Article11. Palladino AA, Bennett MJ, Stanley CA. Hyperinsulinism in infancy and childhood: when an insulin level is not always enough. Clin Chem. 2008; 54:256–263. PMID: 18156285.
Article12. De Leon DD, Stanley CA. Determination of insulin for the diagnosis of hyperinsulinemic hypoglycemia. Best Pract Res Clin Endocrinol Metab. 2013; 27:763–769. PMID: 24275188.
Article13. Arnoux JB, de Lonlay P, Ribeiro MJ, Hussain K, Blankenstein O, Mohnike K, et al. Congenital hyperinsulinism. Early Hum Dev. 2010; 86:287–294. PMID: 20550977.
Article14. van Veen MR, van Hasselt PM, de Sain-van der Velden MG, Verhoeven N, Hofstede FC, de Koning TJ, et al. Metabolic profiles in children during fasting. Pediatrics. 2011; 127:e1021–e1027. PMID: 21422093.
Article15. Vannucci RC, Vannucci SJ. Hypoglycemic brain injury. Semin Neonatol. 2001; 6:147–155. PMID: 11483020.
Article16. Salhab WA, Wyckoff MH, Laptook AR, Perlman JM. Initial hypoglycemia and neonatal brain injury in term infants with severe fetal acidemia. Pediatrics. 2004; 114:361–366. PMID: 15286217.
Article17. Rozance PJ, Hay WW. Hypoglycemia in newborn infants: Features associated with adverse outcomes. Biol Neonate. 2006; 90:74–86. PMID: 16534190.
Article18. Gataullina S, Dellatolas G, Perdry H, Robert JJ, Valayannopoulos V, Touati G, et al. Comorbidity and metabolic context are crucial factors determining neurological sequelae of hypoglycaemia. Dev Med Child Neurol. 2012; 54:1012–1017. PMID: 22924392.
Article19. Ashcroft FM, Rorsman P. K(ATP) channels and islet hormone secretion: new insights and controversies. Nat Rev Endocrinol. 2013; 9:660–669. PMID: 24042324.
Article20. Yorifuji T, Masue M, Nishibori H. Congenital hyperinsulinism: global and Japanese perspectives. Pediatr Int. Forthcoming 2014; http://dx.doi.org/10.1111/ped.12390.21. Pearson ER, Boj SF, Steele AM, Barrett T, Stals K, Shield JP, et al. Macrosomia and hyperinsulinaemic hypoglycaemia in patients with heterozygous mutations in the HNF4A gene. PLoS Med. 2007; 4:e118. PMID: 17407387.
Article22. Kapoor RR, Locke J, Colclough K, Wales J, Conn JJ, Hattersley AT, et al. Persistent hyperinsulinemic hypoglycemia and maturity-onset diabetes of the young due to heterozygous HNF4A mutations. Diabetes. 2008; 57:1659–1663. PMID: 18268044.
Article23. Flanagan SE, Kapoor RR, Mali G, Cody D, Murphy N, Schwahn B, et al. Diazoxide-responsive hyperinsulinemic hypoglycemia caused by HNF4A gene mutations. Eur J Endocrinol. 2010; 162:987–992. PMID: 20164212.
Article24. Stanescu DE, Hughes N, Kaplan B, Stanley CA, De Leon DD. Novel presentations of congenital hyperinsulinism due to mutations in the MODY genes: HNF1A and HNF4A. J Clin Endocrinol Metab. 2012; 97:E2026–E2030. PMID: 22802087.25. Colclough K, Bellanne-Chantelot C, Saint-Martin C, Flanagan SE, Ellard S. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha and 4 alpha in maturity-onset diabetes of the young and hyperinsulinemic hypoglycemia. Hum Mutat. 2013; 34:669–685. PMID: 23348805.
Article26. Snider KE, Becker S, Boyajian L, Shyng SL, MacMullen C, Hughes N, et al. Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J Clin Endocrinol Metab. 2013; 98:E355–E363. PMID: 23275527.
Article27. Kapoor RR, Flanagan SE, Arya VB, Shield JP, Ellard S, Hussain K. Clinical and molecular characterisation of 300 patients with congenital hyperinsulinism. Eur J Endocrinol. 2013; 168:557–564. PMID: 23345197.
Article28. Sempoux C, Guiot Y, Jaubert F, Rahier J. Focal and diffuse forms of congenital hyperinsulinism: the keys for differential diagnosis. Endocr Pathol. 2004; 15:241–246. PMID: 15640550.
Article29. Pinney SE, MacMullen C, Becker S, Lin YW, Hanna C, Thornton P, et al. Clinical characteristics and biochemical mechanisms of congenital hyperinsulinism associated with dominant KATP channel mutations. J Clin Invest. 2008; 118:2877–2886. PMID: 18596924.
Article30. Macmullen CM, Zhou Q, Snider KE, Tewson PH, Becker SA, Aziz AR, et al. Diazoxide-unresponsive congenital hyperinsulinism in children with dominant mutations of the β-cell sulfonylurea receptor SUR1. Diabetes. 2011; 60:1797–1804. PMID: 21536946.
Article31. de Lonlay P, Fournet JC, Rahier J, Gross-Morand MS, Poggi-Travert F, Foussier V, et al. Somatic deletion of the imprinted 11p15 region in sporadic persistent hyperinsulinemic hypoglycemia of infancy is specific of focal adenomatous hyperplasia and endorses partial pancreatectomy. J Clin Invest. 1997; 100:802–807. PMID: 9259578.
Article32. Verkarre V, Fournet JC, de Lonlay P, Gross-Morand MS, Devillers M, Rahier J, et al. Paternal mutation of the sulfonylurea receptor (SUR1) gene and maternal loss of 11p15 imprinted genes lead to persistent hyperinsulinism in focal adenomatous hyperplasia. J Clin Invest. 1998; 102:1286–1291. PMID: 9769320.
Article33. Fournet JC, Mayaud C, de Lonlay P, Gross-Morand MS, Verkarre V, Castanet M, et al. Unbalanced expression of 11p15 imprinted genes in focal forms of congenital hyperinsulinism: association with a reduction to homozygosity of a mutation in ABCC8 or KCNJ11. Am J Pathol. 2001; 158:2177–2184. PMID: 11395395.
Article34. Damaj L, le Lorch M, Verkarre V, Werl C, Hubert L, Nihoul-Fekete C, et al. Chromosome 11p15 paternal isodisomy in focal forms of neonatal hyperinsulinism. J Clin Endocrinol Metab. 2008; 93:4941–4947. PMID: 18796520.
Article35. Suchi M, Thornton PS, Adzick NS, MacMullen C, Ganguly A, Stanley CA, et al. Congenital hyperinsulinism: intraoperative biopsy interpretation can direct the extent of pancreatectomy. Am J Surg Pathol. 2004; 28:1326–1335. PMID: 15371948.36. Suchi M, MacMullen CM, Thornton PS, Adzick NS, Ganguly A, Ruchelli ED, et al. Molecular and immunohistochemical analyses of the focal form of congenital hyperinsulinism. Mod Pathol. 2006; 19:122–129. PMID: 16357843.
Article37. Rahier J, Guiot Y, Sempoux C. Morphologic analysis of focal and diffuse forms of congenital hyperinsulinism. Semin Pediatr Surg. 2011; 20:3–12. PMID: 21185997.
Article38. Otonkoski T, Nanto-Salonen K, Seppanen M, Veijola R, Huopio H, Hussain K, et al. Noninvasive diagnosis of focal hyperinsulinism of infancy with [18F]-DOPA positron emission tomography. Diabetes. 2006; 55:13–18. PMID: 16380471.
Article39. Blomberg BA, Moghbel MC, Saboury B, Stanley CA, Alavi A. The value of radiologic interventions and (18) F-DOPA PET in diagnosing and localizing focal congenital hyperinsulinism: systematic review and meta-analysis. Mol Imaging Biol. 2013; 15:97–105. PMID: 22752652.
Article40. Yang J, Hao R, Zhu X. Diagnostic role of 18F-dihydroxy-phenylalanine positron emission tomography in patients with congenital hyperinsulinism: a meta-analysis. Nucl Med Commun. 2013; 34:347–353. PMID: 23376859.
Article41. Masue M, Nishibori H, Fukuyama S, Yoshizawa A, Okamoto S, Doi R, et al. Diagnostic accuracy of [18F]-fluoro-L-dihydroxyphenylalanine positron emission tomography scan for persistent congenital hyperinsulinism in Japan. Clin Endocrinol (Oxf). 2011; 75:342–346. PMID: 21521340.
Article42. Glaser B, Ryan F, Donath M, Landau H, Stanley CA, Baker L, et al. Hyperinsulinism caused by paternal-specific inheritance of a recessive mutation in the sulfonylurea-receptor gene. Diabetes. 1999; 48:1652–1657. PMID: 10426386.
Article43. De Leon DD, Stanley CA. Mechanisms of disease: advances in diagnosis and treatment of hyperinsulinism in neonates. Nat Clin Pract Endocrinol Metab. 2007; 3:57–68. PMID: 17179930.
Article44. Fernandez-Marmiesse A, Salas A, Vega A, Fernandez-Lorenzo JR, Barreiro J, Carracedo A. Mutation spectra of ABCC8 gene in Spanish patients with Hyperinsulinism of Infancy (HI). Hum Mutat. 2006; 27:214. PMID: 16429405.45. Sandal T, Laborie LB, Brusgaard K, Eide SA, Christesen HB, Sovik O, et al. The spectrum of ABCC8 mutations in Norwegian patients with congenital hyperinsulinism of infancy. Clin Genet. 2009; 75:440–448. PMID: 19475716.
Article46. Yorifuji T, Kawakita R, Nagai S, Sugimine A, Doi H, Nomura A, et al. Molecular and clinical analysis of Japanese patients with persistent congenital hyperinsulinism: predominance of paternally inherited monoallelic mutations in the KATP channel genes. J Clin Endocrinol Metab. 2011; 96:E141–E145. PMID: 20943781.47. Mohnike K, Wieland I, Barthlen W, Vogelgesang S, Empting S, Mohnike W, et al. Clinical and genetic evaluation of patients with KATP channel mutations from the German registry for congenital hyperinsulinism. Horm Res Paediatr. 2014; 81:156–168. PMID: 24401662.
Article48. Su C, Gong C, Sanger P, Li W, Wu D, Gu Y, et al. Long-term follow-up and mutation analysis of 27 chinese cases of congenital hyperinsulinism. Horm Res Paediatr. 2014; 81:169–176. PMID: 24434300.
Article49. Banerjee I, Skae M, Flanagan SE, Rigby L, Patel L, Didi M, et al. The contribution of rapid KATP channel gene mutation analysis to the clinical management of children with congenital hyperinsulinism. Eur J Endocrinol. 2011; 164:733–740. PMID: 21378087.
Article50. Stanley CA, Lieu YK, Hsu BY, Burlina AB, Greenberg CR, Hopwood NJ, et al. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med. 1998; 338:1352–1357. PMID: 9571255.
Article51. Molven A, Matre GE, Duran M, Wanders RJ, Rishaug U, Njolstad PR, et al. Familial hyperinsulinemic hypoglycemia caused by a defect in the SCHAD enzyme of mitochondrial fatty acid oxidation. Diabetes. 2004; 53:221–227. PMID: 14693719.
Article52. Flanagan SE, Patch AM, Locke JM, Akcay T, Simsek E, Alaei M, et al. Genome-wide homozygosity analysis reveals HADH mutations as a common cause of diazoxide-responsive hyperinsulinemic-hypoglycemia in consanguineous pedigrees. J Clin Endocrinol Metab. 2011; 96:E498–E502. PMID: 21252247.53. Heslegrave AJ, Hussain K. Novel insights into fatty acid oxidation, amino acid metabolism, and insulin secretion from studying patients with loss of function mutations in 3-hydroxyacyl-CoA dehydrogenase. J Clin Endocrinol Metab. 2013; 98:496–501. PMID: 23253615.
Article54. Kassem S, Bhandari S, Rodríguez-Bada P, Motaghedi R, Heyman M, Garcia-Gimeno MA, et al. Large islets, beta-cell proliferation, and a glucokinase mutation. N Engl J Med. 2010; 362:1348–1350. PMID: 20375417.
Article55. Barbetti F, Cobo-Vuilleumier N, Dionisi-Vici C, Toni S, Ciampalini P, Massa O, et al. Opposite clinical phenotypes of glucokinase disease: description of a novel activating mutation and contiguous inactivating mutations in human glucokinase (GCK) gene. Mol Endocrinol. 2009; 23:1983–1989. PMID: 19884385.
Article56. Christesen HB, Tribble ND, Molven A, Siddiqui J, Sandal T, Brusgaard K, et al. Activating glucokinase (GCK) mutations as a cause of medically responsive congenital hyperinsulinism: prevalence in children and characterisation of a novel GCK mutation. Eur J Endocrinol. 2008; 159:27–34. PMID: 18450771.
Article57. Osbak KK, Colclough K, Saint-Martin C, Beer NL, Bellanne-Chantelot C, Ellard S, et al. Update on mutations in glucokinase (GCK), which cause maturity-onset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemic hypoglycemia. Hum Mutat. 2009; 30:1512–1526. PMID: 19790256.
Article58. Henquin JC, Sempoux C, Marchandise J, Godecharles S, Guiot Y, Nenquin M, et al. Congenital hyperinsulinism caused by hexokinase I expression or glucokinase-activating mutation in a subset of β-cells. Diabetes. 2013; 62:1689–1696. PMID: 23274908.
Article59. Gonzalez-Barroso MM, Giurgea I, Bouillaud F, Anedda A, Bellanne-Chantelot C, Hubert L, et al. Mutations in UCP2 in congenital hyperinsulinism reveal a role for regulation of insulin secretion. PLoS One. 2008; 3:e3850. PMID: 19065272.
Article60. Otonkoski T, Kaminen N, Ustinov J, Lapatto R, Meissner T, Mayatepek E, et al. Physical exercise-induced hyperinsulinemic hypoglycemia is an autosomal-dominant trait characterized by abnormal pyruvate-induced insulin release. Diabetes. 2003; 52:199–204. PMID: 12502513.
Article61. Kapoor RR, James C, Hussain K. Hyperinsulinism in developmental syndromes. Endocr Dev. 2009; 14:95–113. PMID: 19293578.
Article62. Bitner-Glindzicz M, Lindley KJ, Rutland P, Blaydon D, Smith VV, Milla PJ, et al. A recessive contiguous gene deletion causing infantile hyperinsulinism, enteropathy and deafness identifies the Usher type 1C gene. Nat Genet. 2000; 26:56–60. PMID: 10973248.
Article63. Al Mutair AN, Brusgaard K, Bin-Abbas B, Hussain K, Felimban N, Al Shaikh A, et al. Heterogeneity in phenotype of usher-congenital hyperinsulinism syndrome: hearing loss, retinitis pigmentosa, and hyperinsulinemic hypoglycemia ranging from severe to mild with conversion to diabetes. Diabetes Care. 2013; 36:557–561. PMID: 23150283.64. Touati G, Poggi-Travert F, Ogier de Baulny H, Rahier J, Brunelle F, Nihoul-Fekete C, et al. Long-term treatment of persistent hyperinsulinaemic hypoglycaemia of infancy with diazoxide: a retrospective review of 77 cases and analysis of efficacy-predicting criteria. Eur J Pediatr. 1998; 157:628–633. PMID: 9727845.
Article65. Demirel F, Unal S, Cetin II, Esen I, Arasli A. Pulmonary hypertension and reopening of the ductus arteriosus in an infant treated with diazoxide. J Pediatr Endocrinol Metab. 2011; 24:603–605. PMID: 21932611.
Article66. Yoshida K, Kawai M, Marumo C, Kanazawa H, Matsukura T, Kusuda S, et al. High prevalence of severe circulatory complications with diazoxide in premature infants. Neonatology. 2014; 105:166–171. PMID: 24458138.
Article67. Glaser B, Hirsch HJ, Landau H. Persistent hyperinsulinemic hypoglycemia of infancy: long-term octreotide treatment without pancreatectomy. J Pediatr. 1993; 123:644–650. PMID: 8410523.
Article68. Thornton PS, Alter CA, Katz LE, Baker L, Stanley CA. Short- and long-term use of octreotide in the treatment of congenital hyperinsulinism. J Pediatr. 1993; 123:637–643. PMID: 8410522.
Article69. Yorifuji T, Kawakita R, Hosokawa Y, Fujimaru R, Matsubara K, Aizu K, et al. Efficacy and safety of long-term, continuous subcutaneous octreotide infusion for patients with different subtypes of KATP-channel hyperinsulinism. Clin Endocrinol (Oxf). 2013; 78:891–897. PMID: 23067144.
Article70. Koren I, Riskin A, Barthlen W, Gillis D. Hepatitis in an infant treated with octreotide for congenital hyperinsulinism. J Pediatr Endocrinol Metab. 2013; 26:183–185. PMID: 23327817.
Article71. Laje P, Halaby L, Adzick NS, Stanley CA. Necrotizing enterocolitis in neonates receiving octreotide for the management of congenital hyperinsulinism. Pediatr Diabetes. 2010; 11:142–147. PMID: 19558634.
Article72. Celik N, Cinaz P, Emeksiz HC, Hussain K, Camurdan O, Bideci A, et al. Octreotide-induced long QT syndrome in a child with congenital hyperinsulinemia and a novel missense mutation (p.Met115Val) in the ABCC8 gene. Horm Res Paediatr. 2013; 80:299–303. PMID: 24080777.
Article73. Mohnike K, Blankenstein O, Pfuetzner A, Potzsch S, Schober E, Steiner S, et al. Long-term non-surgical therapy of severe persistent congenital hyperinsulinism with glucagon. Horm Res. 2008; 70:59–64. PMID: 18493152.
Article74. Neylon OM, Moran MM, Pellicano A, Nightingale M, O'Connell MA. Successful subcutaneous glucagon use for persistent hypoglycaemia in congenital hyperinsulinism. J Pediatr Endocrinol Metab. 2013; 26:1157–1161. PMID: 23813352.
Article75. von Rohden L, Mohnike K, Mau H, Eberhard T, Mohnike W, Blankenstein O, et al. Visualization of the focus in congenital hyperinsulinism by intraoperative sonography. Semin Pediatr Surg. 2011; 20:28–31. PMID: 21186001.
Article76. Adzick NS, Thornton PS, Stanley CA, Kaye RD, Ruchelli E. A multidisciplinary approach to the focal form of congenital hyperinsulinism leads to successful treatment by partial pancreatectomy. J Pediatr Surg. 2004; 39:270–275. PMID: 15017536.
Article77. Palladino AA, Stanley CA. A specialized team approach to diagnosis and medical versus surgical treatment of infants with congenital hyperinsulinism. Semin Pediatr Surg. 2011; 20:32–37. PMID: 21186002.
Article78. Laje P, Stanley CA, Palladino AA, Becker SA, Adzick NS. Pancreatic head resection and Roux-en-Y pancreaticojejunostomy for the treatment of the focal form of congenital hyperinsulinism. J Pediatr Surg. 2012; 47:130–135. PMID: 22244405.
Article79. Barthlen W, de Lonlay P. Congenital hyperinsulinism. Semin Pediatr Surg. 2011; 20:1–2. PMID: 21185996.80. Barthlen W. Surgery in congenital hyperinsulinism-tips and tricks not only for surgeons: a practical guide. Semin Pediatr Surg. 2011; 20:56–59. PMID: 21186007.
Article81. Proverbio MC, Mangano E, Gessi A, Bordoni R, Spinelli R, Asselta R, et al. Whole genome SNP genotyping and exome sequencing reveal novel genetic variants and putative causative genes in congenital hyperinsulinism. PLoS One. 2013; 8:e68740. PMID: 23869231.
Article82. Flanagan SE, Xie W, Caswell R, Damhuis A, Vianey-Saban C, Akcay T, et al. Next-generation sequencing reveals deep intronic cryptic ABCC8 and HADH splicing founder mutations causing hyperinsulinism by pseudoexon activation. Am J Hum Genet. 2013; 92:131–136. PMID: 23273570.
Article83. Thompson LP, Al-Hasan Y. Impact of oxidative stress in fetal programming. J Pregnancy. 2012; 2012:582748. PMID: 22848830.
Article84. Heinis M, Simon MT, Ilc K, Mazure NM, Pouyssegur J, Scharfmann R, et al. Oxygen tension regulates pancreatic beta-cell differentiation through hypoxia-inducible factor 1alpha. Diabetes. 2010; 59:662–669. PMID: 20009089.
Article85. Hakim F, Kaitsuka T, Raeed JM, Wei FY, Shiraki N, Akagi T, et al. High oxygen condition facilitates the differentiation of mouse and human pluripotent stem cells into pancreatic progenitors and insulin-producing cells. J Biol Chem. 2014; 289:9623–9638. PMID: 24554704.
Article86. Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, et al. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA. 2014; 311:587–596. PMID: 24519298.
Article87. Mazor-Aronovitch K, Landau H, Gillis D. Surgical versus non-surgical treatment of congenital hyperinsulinism. Pediatr Endocrinol Rev. 2009; 6:424–430. PMID: 19396028.88. Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes. 2000; 49:1325–1333. PMID: 10923633.
Article89. Yorifuji T, Hosokawa Y, Fujimaru R, Kawakita R, Doi H, Matsumoto T, et al. Lasting 18F-DOPA PET uptake after clinical remission of the focal form of congenital hyperinsulinism. Horm Res Paediatr. 2011; 76:286–290. PMID: 21912073.
Article90. Modan-Moses D, Koren I, Mazor-Aronovitch K, Pinhas-Hamiel O, Landau H. Treatment of congenital hyperinsulinism with lanreotide acetate (Somatuline Autogel). J Clin Endocrinol Metab. 2011; 96:2312–2317. PMID: 21697252.
Article91. Kuhnen P, Marquard J, Ernert A, Meissner T, Raile K, Wannenmacher G, et al. Long-term lanreotide treatment in six patients with congenital hyperinsulinism. Horm Res Paediatr. 2012; 78:106–112. PMID: 22907123.
Article92. Le Quan Sang KH, Arnoux JB, Mamoune A, Saint-Martin C, Bellanne-Chantelot C, Valayannopoulos V, et al. Successful treatment of congenital hyperinsulinism with long-acting release octreotide. Eur J Endocrinol. 2012; 166:333–339. PMID: 22048969.
Article93. de Heide LJ, Laskewitz AJ, Apers JA. Treatment of severe postRYGB hyperinsulinemic hypoglycemia with pasireotide: a comparison with octreotide on insulin, glucagon, and GLP-1. Surg Obes Relat Dis. 2013; 12. 04:[Epub]. http://dx.doi.org/10.1016/j.soard.2013.11.006.
Article94. Feelders RA, de Herder WW, Neggers SJ, van der Lely AJ, Hofland LJ. Pasireotide, a multi-somatostatin receptor ligand with potential efficacy for treatment of pituitary and neuroendocrine tumors. Drugs Today (Barc). 2013; 49:89–103. PMID: 23462624.95. Martin GM, Chen PC, Devaraneni P, Shyng SL. Pharmacological rescue of trafficking-impaired ATP-sensitive potassium channels. Front Physiol. 2013; 4:386. PMID: 24399968.
Article96. Yan FF, Casey J, Shyng SL. Sulfonylureas correct trafficking defects of disease-causing ATP-sensitive potassium channels by binding to the channel complex. J Biol Chem. 2006; 281:33403–33413. PMID: 16956886.
Article97. Chen PC, Olson EM, Zhou Q, Kryukova Y, Sampson HM, Thomas DY, et al. Carbamazepine as a novel small molecule corrector of trafficking-impaired ATP-sensitive potassium channels identified in congenital hyperinsulinism. J Biol Chem. 2013; 288:20942–20954. PMID: 23744072.
Article98. Calabria AC, Li C, Gallagher PR, Stanley CA, De Leon DD. GLP-1 receptor antagonist exendin-(9-39) elevates fasting blood glucose levels in congenital hyperinsulinism owing to inactivating mutations in the ATP-sensitive K+ channel. Diabetes. 2012; 61:2585–2591. PMID: 22855730.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Laparoscopic Surgery for Advanced Gastric Cancer: Current Status and Future Perspectives
- A Case of Hyperinsulinism/hyperammonemia Syndrome
- Erratum: Leptospirosis in the Republic of Korea: Historical Perspectives, Current Status and Future Challenges
- Use of Stem Cell in Fetal Therapy: Current Status and Future Perspectives
- A Case of 2-Month-Old Infant with Persistent Hyperinsulinemic Hypoglycemia Presenting as Atonic Seizure