Ann Hepatobiliary Pancreat Surg.
2021 Nov;25(4):451-455. 10.14701/ahbps.2021.25.4.451.
Paradigm shift for defining the resectability of pancreatic cancer
- Affiliations
-
- 1Department of Surgery, Center for Liver and Pancreato-Biliary Cancer, National Cancer Center, Goyang, Korea
- KMID: 2523047
- DOI: http://doi.org/10.14701/ahbps.2021.25.4.451
Abstract
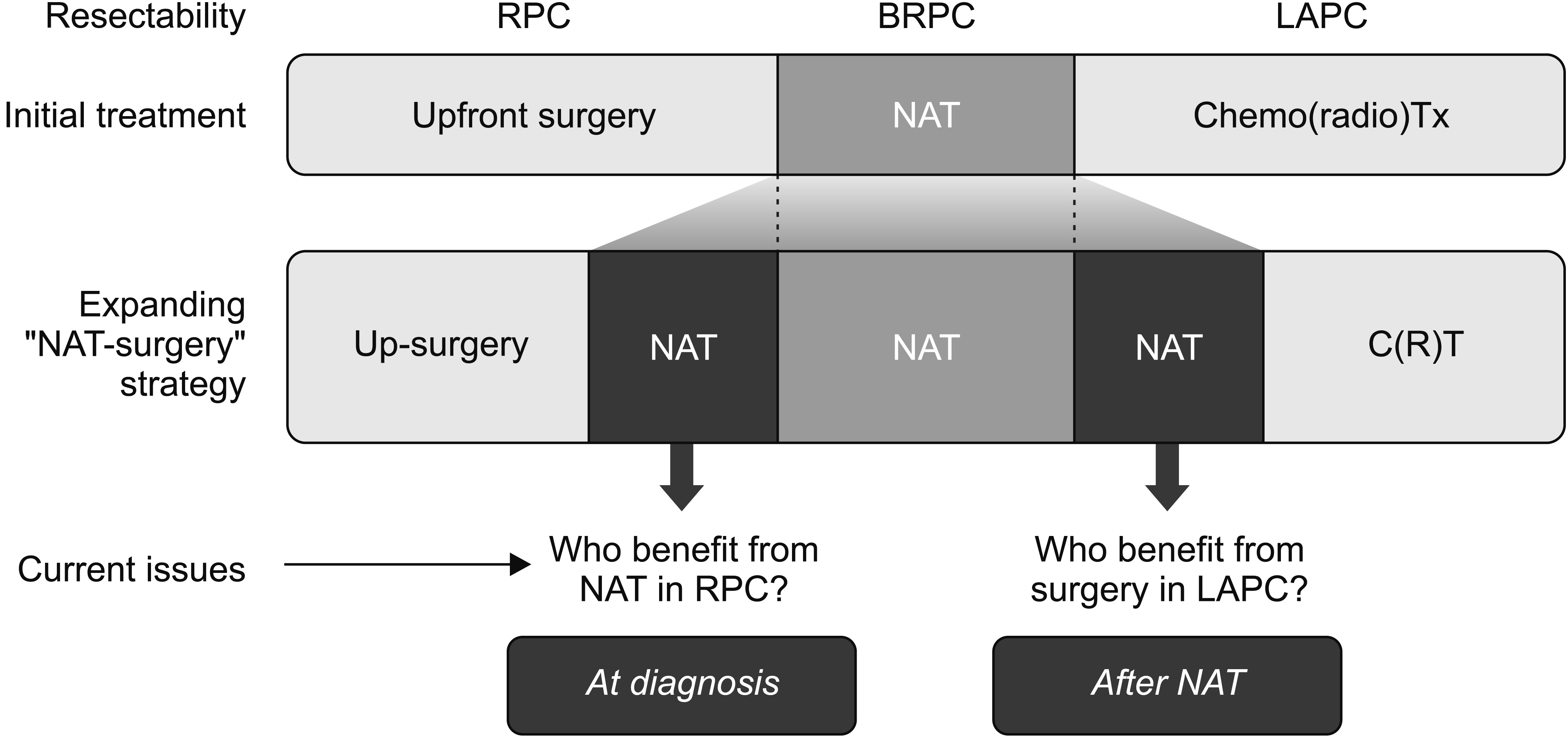
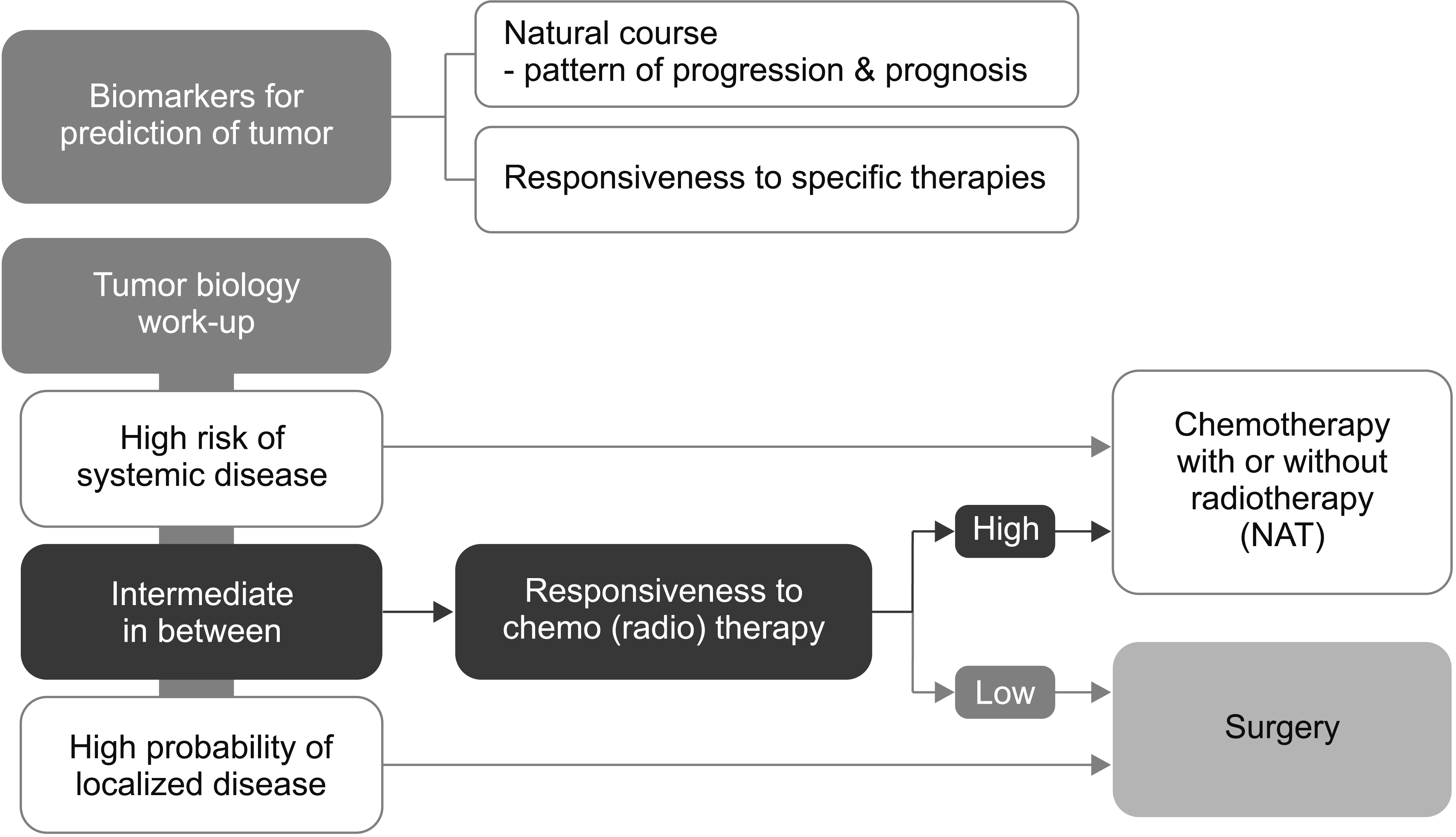
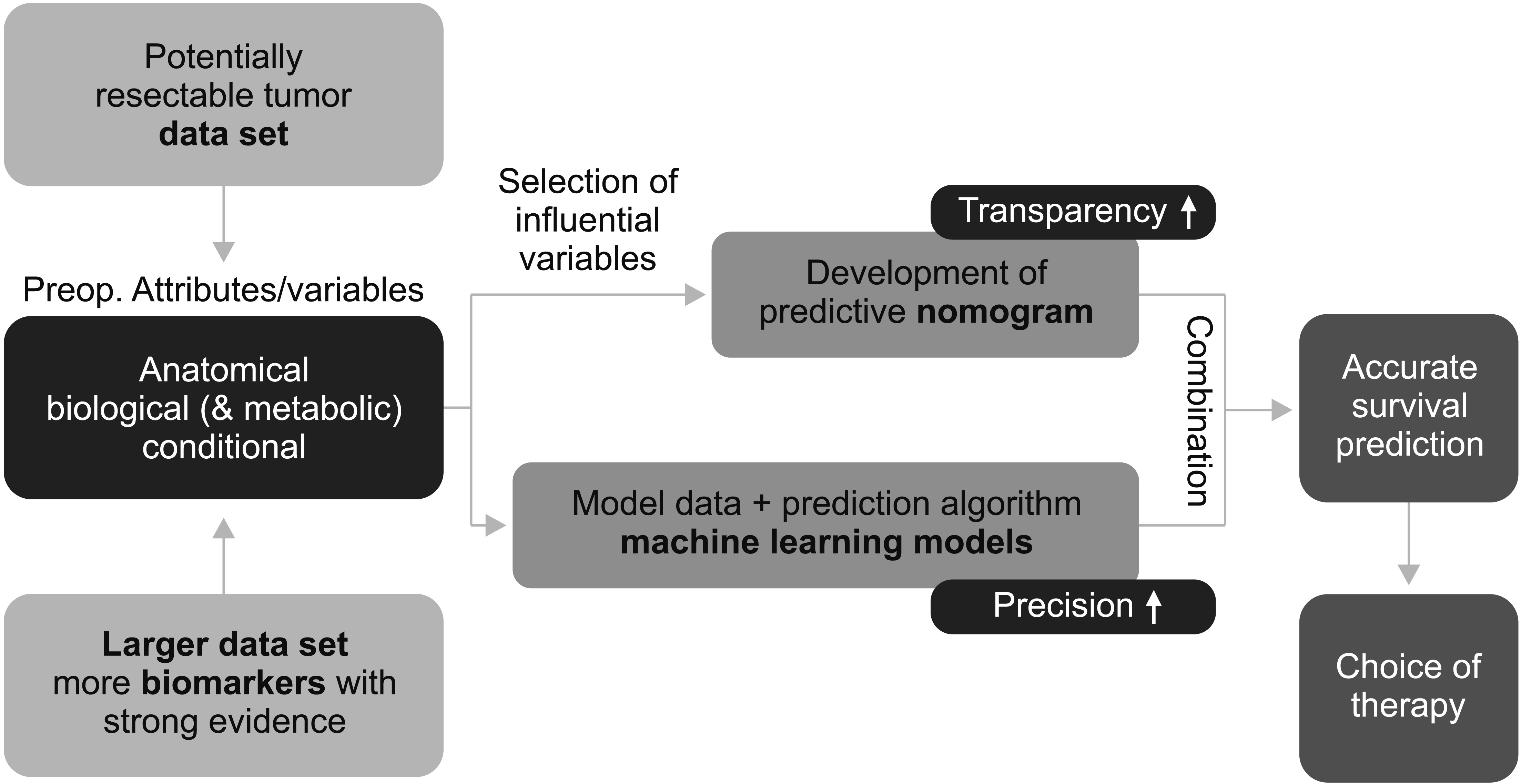
- Supported by the expanding indications for neoadjuvant therapy (NAT) for advanced pancreatic cancer (PC), the concept of resectability has evolved from being mostly based on the anatomical tumor extent to considering the biological and conditional factors relevant to prognosis. Therefore, it is more reasonable to define the “criteria for surgical resection” instead of using the “(technical) resectability criteria.” NAT has been used in resectable PCs (RPC) with a high risk of early systemic recurrence, as predicted by various biological or anatomical markers. Moreover, the indications for NAT followed by conversion surgery or adjuvant surgery for borderline resectable or locally advanced PC (LAPC) are gradually expanding. Therefore, it is important to define the RPC group that will benefit from NAT and the LAPC group that will benefit from post-NAT surgery. At diagnosis, population-based approaches, such as prognostic stratification and staging systems and personalized outcome-based approaches using prognostic prediction models can be used to determine the criteria for treatment options. Standardized indications for conversion surgery are needed for patients who are initially treated with NAT. In addition to imaging-based morphological criteria, biological criteria, including CA19-9, and various metabolic criteria can be used to establish predicted outcome-based criteria. Multicenter collaboration is required to develop a large database with standardized data collection for various biomarkers and response data after NAT to establish more accurate outcome prediction models to define the new resectability criteria.
Figure
Reference
-
1. National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology: pancreatic adenocarcinoma, version 2 [Internet]. Plymouth Meeting: NCCN 2021. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455. cited 2021 Jun 5. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455.2. Ishido K, Hakamada K, Kimura N, Miura T, Wakiya T. Essential updates 2018/2019: current topics in the surgical treatment of pancreatic ductal adenocarcinoma. Ann Gastroenterol Surg. 2020; 5:7–23. DOI: 10.1002/ags3.12379. PMID: 33532676. PMCID: PMC7832965.
Article3. Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, et al. 2014; Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 155:977–988. DOI: 10.1016/j.surg.2014.02.001. PMID: 24856119.
Article4. Cady B. 1997; Basic principles in surgical oncology. Arch Surg. 132:338–346. DOI: 10.1001/archsurg.1997.01430280012001. PMID: 9108752.
Article5. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. 2018; FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 379:2395–2406. DOI: 10.1056/NEJMoa1809775. PMID: 30575490.
Article6. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. 2017; Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 389:1011–1024. DOI: 10.1016/S0140-6736(16)32409-6. PMID: 28129987.
Article7. Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH, et al. 2018; Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg. 268:215–222. DOI: 10.1097/SLA.0000000000002705. PMID: 29462005.8. Klaiber U, Schnaidt ES, Hinz U, Gaida MM, Heger U, Hank T, et al. 2021; Prognostic factors of survival after neoadjuvant treatment and resection for initially unresectable pancreatic cancer. Ann Surg. 273:154–162. DOI: 10.1097/SLA.0000000000003270. PMID: 30921051.
Article9. Kato H, Horiguchi A, Ito M, Asano Y, Arakawa S. Essential updates 2019/2020: multimodal treatment of localized pancreatic adenocarcinoma: current topics and updates in survival outcomes and prognostic factors. Ann Gastroenterol Surg. 2021; 5:132–151. DOI: 10.1002/ags3.12427. PMID: 33860134. PMCID: PMC8034700.
Article10. Isaji S, Mizuno S, Windsor JA, Bassi C, Fernández-Del Castillo C, Hackert T, et al. 2018; International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 18:2–11. DOI: 10.1016/j.pan.2017.11.011. PMID: 29191513.
Article11. Moon D, Kim H, Han Y, Byun Y, Choi Y, Kang J, et al. Preoperative carbohydrate antigen 19-9 and standard uptake value of positron emission tomography-computed tomography as prognostic markers in patients with pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci. https://doi.org/10.1002/jhbp.845 [in press]. 2020; DOI: 10.1002/jhbp.845. PMID: 33063453.
Article12. Dreyer SB, Pinese M, Jamieson NB, Scarlett CJ, Colvin EK, Pajic M, et al. 2020; Precision oncology in surgery: patient selection for operable pancreatic cancer. Ann Surg. 272:366–376. DOI: 10.1097/SLA.0000000000003143. PMID: 32675551. PMCID: PMC7373491.13. Hu H, Zhu Y, Pu N, Burkhart RA, Burns W, Laheru D, et al. 2020; Association of germline variants in human DNA damage repair genes and response to adjuvant chemotherapy in resected pancreatic ductal adenocarcinoma. J Am Coll Surg. 231:527–535.e14. DOI: 10.1016/j.jamcollsurg.2020.06.019. PMID: 32659497.
Article14. Gemenetzis G, Groot VP, Yu J, Ding D, Teinor JA, Javed AA, et al. 2018; Circulating tumor cells dynamics in pancreatic adenocarcinoma correlate with disease status: results of the prospective CLUSTER study. Ann Surg. 268:408–420. DOI: 10.1097/SLA.0000000000002925. PMID: 30080739.
Article15. Heredia-Soto V, Rodríguez-Salas N, Feliu J. 2021; Liquid biopsy in pancreatic cancer: are we ready to apply it in the clinical practice? Cancers (Basel). 13:1986. DOI: 10.3390/cancers13081986. PMID: 33924143. PMCID: PMC8074327.
Article16. Abunahel BM, Pontre B, Kumar H, Petrov MS. 2021; Pancreas image mining: a systematic review of radiomics. Eur Radiol. 31:3447–3467. DOI: 10.1007/s00330-020-07376-6. PMID: 33151391.
Article17. Kim MK, Woo SM, Park B, Yoon KA, Kim YH, Joo J, et al. 2018; Prognostic implications of multiplex detection of KRAS mutations in Cell-Free DNA from patients with pancreatic ductal adenocarcinoma. Clin Chem. 64:726–734. DOI: 10.1373/clinchem.2017.283721. PMID: 29352043.18. Tas F, Sen F, Odabas H, Kılıc L, Keskın S, Yıldız I. 2013; Performance status of patients is the major prognostic factor at all stages of pancreatic cancer. Int J Clin Oncol. 18:839–846. DOI: 10.1007/s10147-012-0474-9. PMID: 22996141.
Article19. Oba A, Croce C, Hosokawa P, Meguid C, Torphy RJ, Al-Musawi MH, et al. Prognosis based definition of resectability in pancreatic cancer: a road map to new guidelines. https://doi.org/10.1097/SLA.0000000000003859 [in press]. Ann Surg. 2020; DOI: 10.1097/SLA.0000000000003859. PMID: 32149822.
Article20. Ozola Zalite I, Zykus R, Francisco Gonzalez M, Saygili F, Pukitis A, Gaujoux S, et al. 2015; Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: a systematic review. Pancreatology. 15:19–24. DOI: 10.1016/j.pan.2014.11.006. PMID: 25524484.
Article21. Kim N, Han IW, Ryu Y, Hwang DW, Heo JS, Choi DW, et al. 2020; Predictive nomogram for early recurrence after pancreatectomy in resectable pancreatic cancer: risk classification using preoperative clinicopathologic factors. Cancers (Basel). 12:137. DOI: 10.3390/cancers12010137. PMID: 31935830. PMCID: PMC7016958.
Article22. Reames BN, Blair AB, Krell RW, Groot VP, Gemenetzis G, Padussis JC, et al. 2021; Management of locally advanced pancreatic cancer: results of an international survey of current practice. Ann Surg. 273:1173–1181. DOI: 10.1097/SLA.0000000000003568. PMID: 31449138.23. Rangelova E, Wefer A, Persson S, Valente R, Tanaka K, Orsini N, et al. 2021; Surgery improves survival after neoadjuvant therapy for borderline and locally advanced pancreatic cancer: a single institution experience. Ann Surg. 273:579–586. DOI: 10.1097/SLA.0000000000003301. PMID: 30946073.24. Michelakos T, Pergolini I, Castillo CF, Honselmann KC, Cai L, Deshpande V, et al. 2019; Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg. 269:733–740. DOI: 10.1097/SLA.0000000000002600. PMID: 29227344.
Article25. Gemenetzis G, Groot VP, Blair AB, Laheru DA, Zheng L, Narang AK, et al. 2019; Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg. 270:340–347. DOI: 10.1097/SLA.0000000000002753. PMID: 29596120. PMCID: PMC6985003.
Article26. Byun Y, Han Y, Kang JS, Choi YJ, Kim H, Kwon W, et al. 2019; Role of surgical resection in the era of FOLFIRINOX for advanced pancreatic cancer. J Hepatobiliary Pancreat Sci. 26:416–425. DOI: 10.1002/jhbp.648. PMID: 31218836.
Article27. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. 2009; New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. DOI: 10.1016/j.ejca.2008.10.026. PMID: 19097774.
Article28. Wahl RL, Jacene H, Kasamon Y, Lodge MA. 2009; From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 50 Suppl 1:122S–150S. DOI: 10.2967/jnumed.108.057307. PMID: 19403881. PMCID: PMC2755245.
Article29. Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, et al. 1999; Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET study group. Eur J Cancer. 35:1773–1782. DOI: 10.1016/S0959-8049(99)00229-4. PMID: 10673991.30. Truty MJ, Kendrick ML, Nagorney DM, Smoot RL, Cleary SP, Graham RP, et al. 2021; Factors predicting response, perioperative outcomes, and survival following total neoadjuvant therapy for borderline/locally advanced pancreatic cancer. Ann Surg. 273:341–349. DOI: 10.1097/SLA.0000000000003284. PMID: 30946090.
Article31. Tsai S, George B, Wittmann D, Ritch PS, Krepline AN, Aldakkak M, et al. 2020; Importance of normalization of CA19-9 levels following neoadjuvant therapy in patients with localized pancreatic cancer. Ann Surg. 271:740–747. DOI: 10.1097/SLA.0000000000003049. PMID: 30312198.
Article32. Ushida Y, Inoue Y, Ito H, Oba A, Mise Y, Ono Y, et al. 2021; High CA19-9 level in resectable pancreatic cancer is a potential indication of neoadjuvant treatment. Pancreatology. 21:130–137. DOI: 10.1016/j.pan.2020.11.026. PMID: 33303373.
Article33. Ye C, Sadula A, Ren S, Guo X, Yuan M, Yuan C, et al. 2020; The prognostic value of CA19-9 response after neoadjuvant therapy in patients with pancreatic cancer: a systematic review and pooled analysis. Cancer Chemother Pharmacol. 86:731–740. DOI: 10.1007/s00280-020-04165-2. PMID: 33047181.
Article34. Liu H, Zenati MS, Rieser CJ, Al-Abbas A, Lee KK, Singhi AD, et al. 2020; CA19-9 change during neoadjuvant therapy may guide the need for additional adjuvant therapy following resected pancreatic cancer. Ann Surg Oncol. 27:3950–3960. DOI: 10.1245/s10434-020-08468-9. PMID: 32318949. PMCID: PMC7931260.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Imaging in Current Treatment Strategies for Pancreatic Adenocarcinoma
- Radiologic Evaluation for Resectability of Pancreatic Adenocarcinoma
- Diagnostic value of computed tomography in pancreatic cancer
- Prophylaxis of Venous Thromboembolism in Patients with Pancreatic Cancer
- A Clinical Study on Pancreatic Head Cancer