Int J Thyroidol.
2021 Nov;14(2):152-169. 10.11106/ijt.2021.14.2.152.
Cooperative Subtype Switch of Thyroid Hormone Receptor and Nuclear Receptor Corepressor Related Epithelial–Mesenchymal Transition in Papillary Thyroid Cancer
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Surgery, Eulji University School of Medicine, Daejeon, Korea
- 3Department of Surgery, Severance Hospital, Yonsei Cancer Center, Yonsei University College of Medicine , Seoul, Korea
- KMID: 2522920
- DOI: http://doi.org/10.11106/ijt.2021.14.2.152
Abstract
- Background and Objectives
Although thyroid hormones affect human cancer progression, the regulatory mechanism of thyroid hormone receptors in carcinogenesis has not been elucidated. This study aimed to evaluate the expression pattern of the thyroid hormone receptor (TR) and its corepressors, and to investigate the clinical and biological functions of TR.
Materials and Methods
Transcriptomic and clinical data for thyroid cancer were downloaded from The Cancer Genome Atlas. Paraffin-embedded tissue sections from patients who underwent thyroidectomy were used for immunohistochemistry. BCPAP cells were treated with T3 to investigate the thyroid hormone target genes. Thyroid hormone receptor alpha (THRA) and Thyroid hormone receptor beta (THRB) were knocked down by transient siRNA transfection.
Results
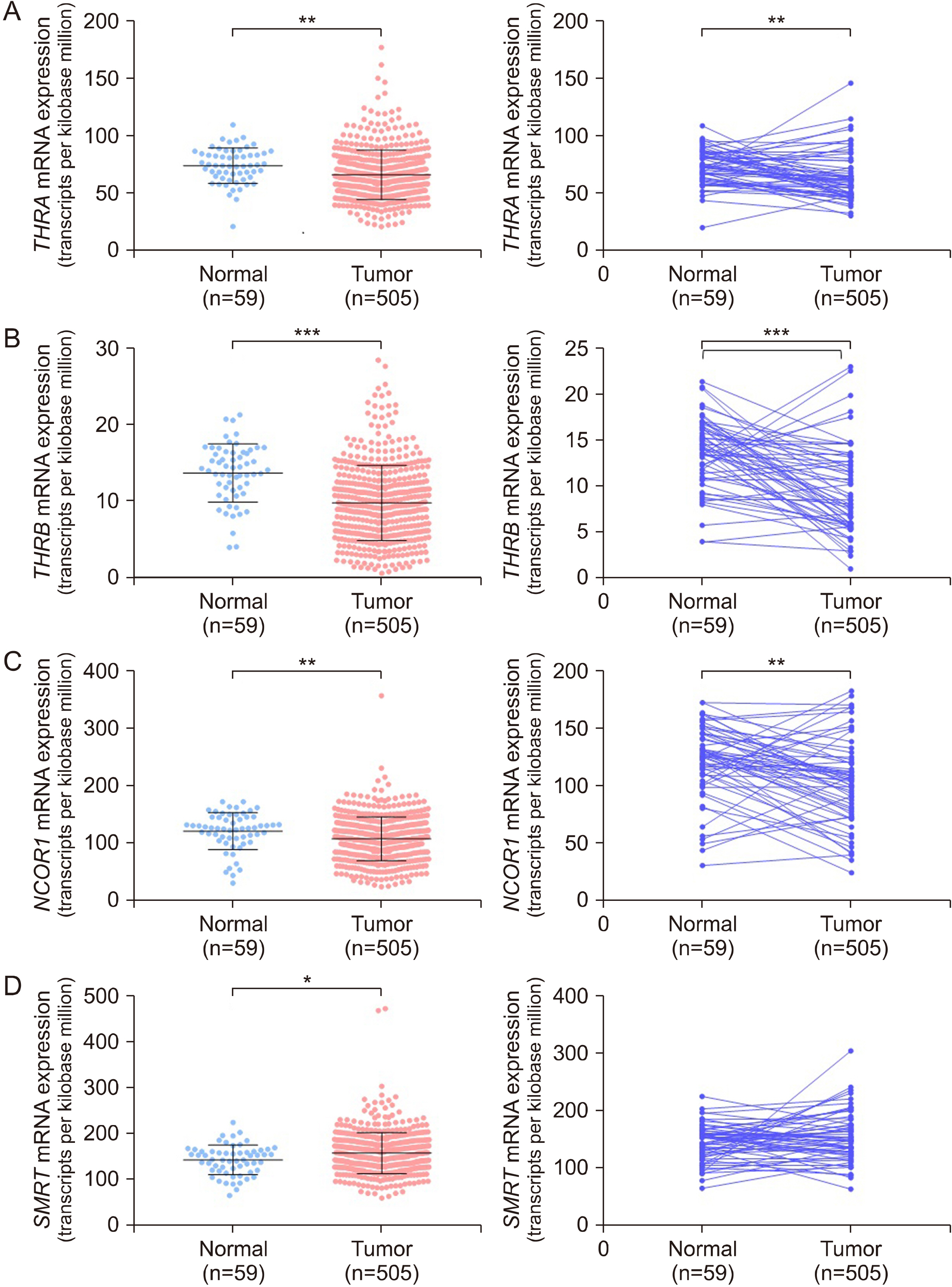
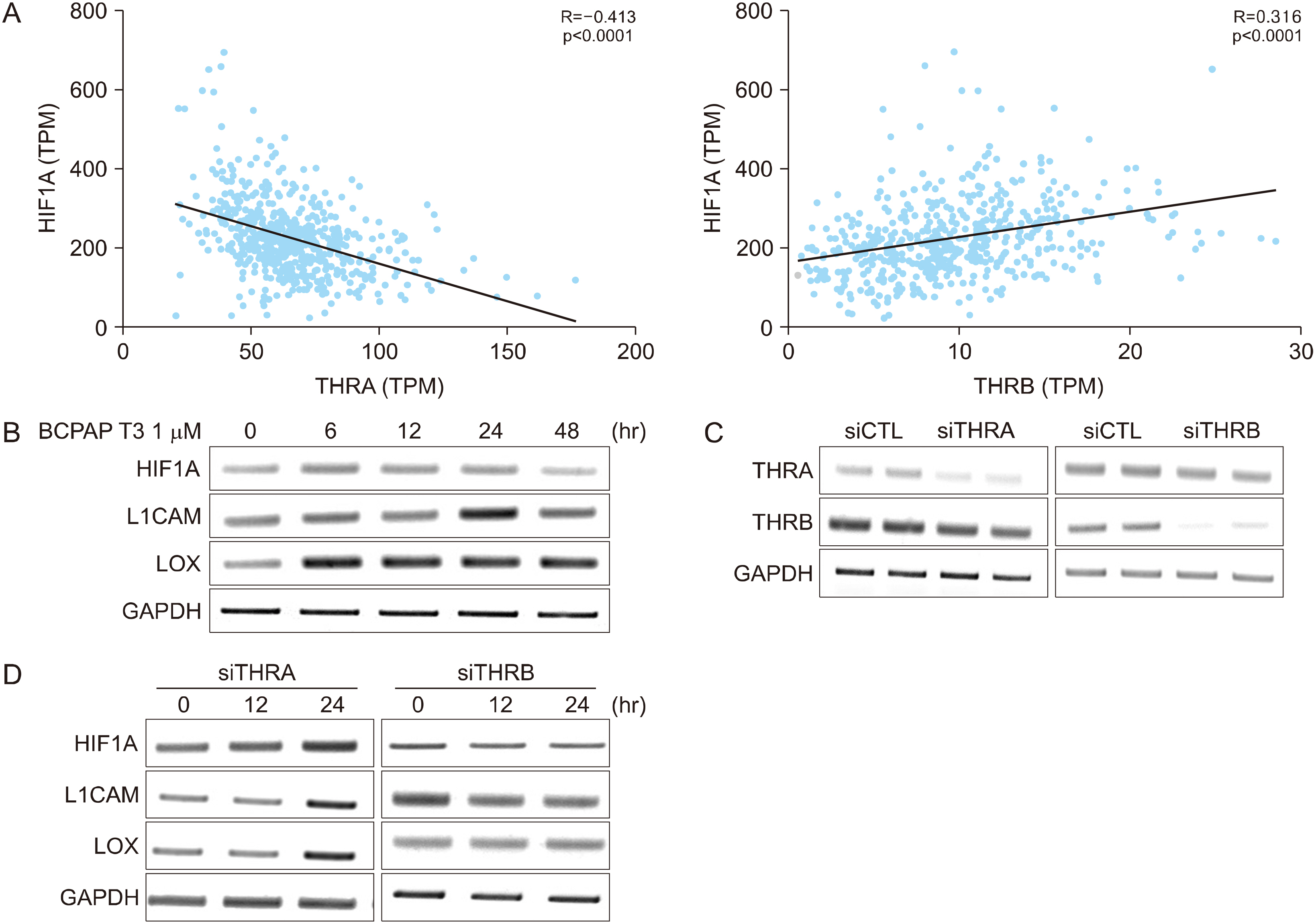
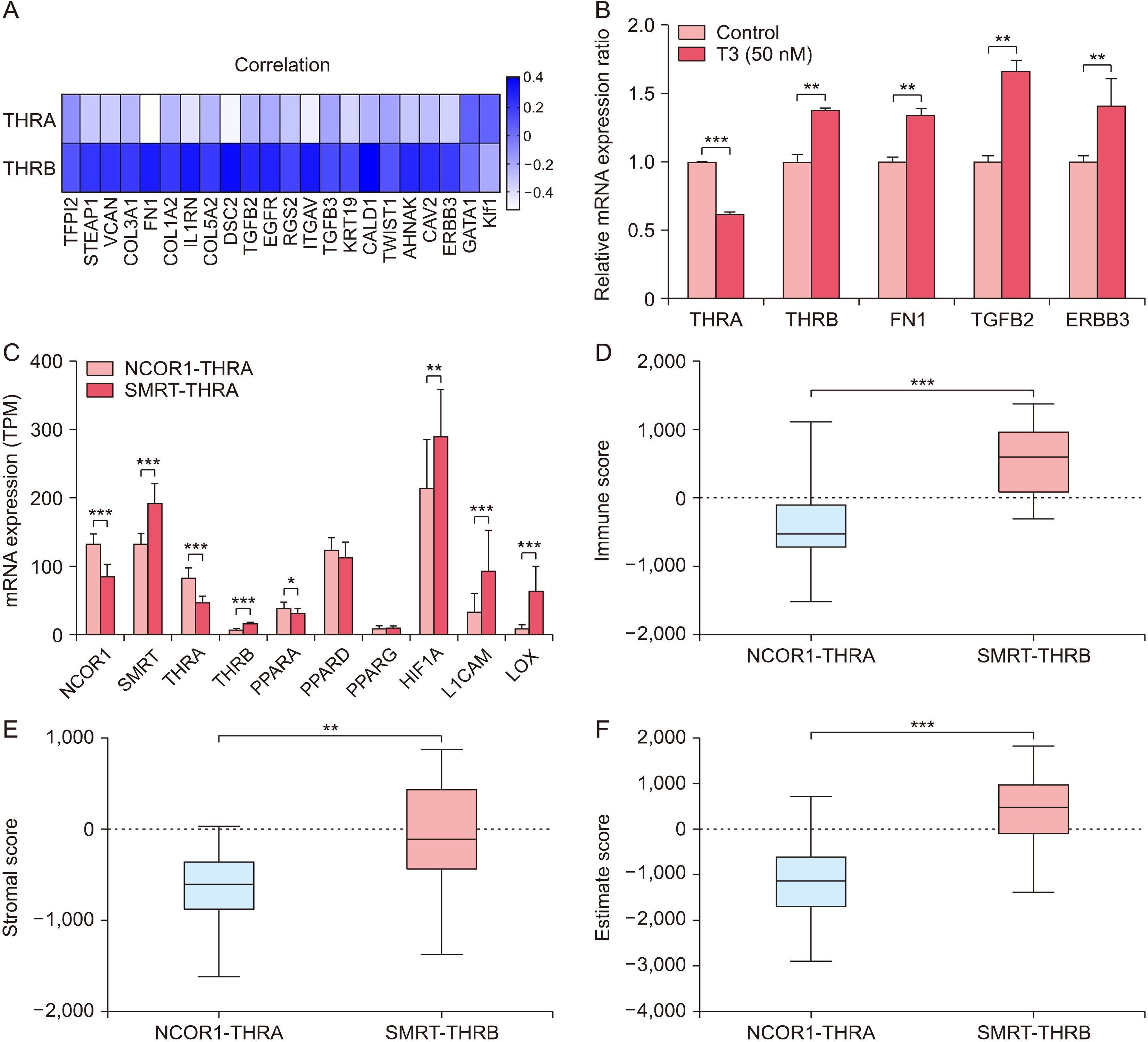
THRA and THRB expression was lower in thyroid cancer tissues than in normal tissues. However, strong focal staining of TRβ was observed in the invasive front. High THRB expression was associated with high Silencing Mediator for Retinoid or Thyroid hormone receptor (SMRT) expression, older age, a high MACIS (distant Metastasis, patient Age, Completeness of resection, local Invasion, and tumor Size) score, more aggressive histological subtypes, more frequent extra-thyroidal extension, and advanced TNM stage. THRB expression was positively correlated with Hypoxia Inducible Factor 1 Subunit Alpha (HIF1A), L1 Cell Adhesion Molecule (L1CAM), and Lysyl Oxidase (LOX) expression. Thyroid hormone-induced HIF1A, L1CAM, and LOX upregulation was abolished by siTHRB but not siTHRA in BCPAP cells. High SMRT and high THRB groups (SMRT/THRB) presented more aggressive clinical features and showed an upregulation of HIF1A, L1CAM, and LOX, as well as of epithelial-mesenchymal transition (EMT)-related genes, causing changes in the tumor microenvironment.
Conclusion
Cooperative subtype switching from NCOR1/THRA to SMRT/THRB was thus related to aggressive clinical and molecular features, possibly related to EMT and EMT-related tumor microenvironment.
Keyword
Figure
Reference
-
References
1. Harvey CB, Williams GR. 2002; Mechanism of thyroid hormone action. Thyroid. 12(6):441–6. DOI: 10.1089/105072502760143791. PMID: 12165104.
Article2. Brent GA. Tissue-specific actions of thyroid hormone: insights from animal models. Rev Endocr Metab Disord. 2000; 1(1-2):27–33. DOI: 10.1023/a:1010056202122. PMID: 11704989.3. Ortiga-Carvalho TM, Sidhaye AR, Wondisford FE. 2014; Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat Rev Endocrinol. 10(10):582–91. DOI: 10.1038/nrendo.2014.143. PMID: 25135573. PMCID: PMC4578869.
Article4. Yen PM. 2001; Physiological and molecular basis of thyroid hormone action. Physiol Rev. 81(3):1097–142. DOI: 10.1152/physrev.2001.81.3.1097. PMID: 11427693.
Article5. Mottis A, Mouchiroud L, Auwerx J. 2013; Emerging roles of the corepressors NCoR1 and SMRT in homeostasis. Genes Dev. 27(8):819–35. DOI: 10.1101/gad.214023.113. PMID: 23630073. PMCID: PMC3650221.6. Jo YS, Ryu D, Maida A, Wang X, Evans RM, Schoonjans K, et al. 2015; Phosphorylation of the nuclear receptor corepressor 1 by protein kinase B switches its corepressor targets in the liver in mice. Hepatology. 62(5):1606–18. DOI: 10.1002/hep.27907. PMID: 25998209. PMCID: PMC4618256.
Article7. Karpen SJ. 2015; Nuancing insulin's actions in liver through rapid NCOR1 phosphorylation. Hepatology. 62(5):1344–5. DOI: 10.1002/hep.28014. PMID: 26223211.8. Shimizu H, Astapova I, Ye F, Bilban M, Cohen RN, Hollenberg AN. 2015; NCoR1 and SMRT play unique roles in thyroid hormone action in vivo. Mol Cell Biol. 35(3):555–65. DOI: 10.1128/MCB.01208-14. PMID: 25421714. PMCID: PMC4285426.9. Tran TV, Kitahara CM, de Vathaire F, Boutron-Ruault MC, Journy N. 2020; Thyroid dysfunction and cancer incidence: a systematic review and meta-analysis. Endocr Relat Cancer. 27(4):245–59. DOI: 10.1530/ERC-19-0417. PMID: 32045361.
Article10. Zhou W, Brumpton B, Kabil O, Gudmundsson J, Thorleifsson G, Weinstock J, et al. 2020; GWAS of thyroid stimulating hormone highlights pleiotropic effects and inverse association with thyroid cancer. Nat Commun. 11(1):3981. DOI: 10.1038/s41467-020-17718-z. PMID: 32769997. PMCID: PMC7414135.
Article11. Yuan S, Kar S, Vithayathil M, Carter P, Mason AM, Burgess S, et al. 2020; Causal associations of thyroid function and dysfunction with overall, breast and thyroid cancer: a two-sample Mendelian randomization study. Int J Cancer. 147(7):1895–903. DOI: 10.1002/ijc.32988. PMID: 32215913. PMCID: PMC7611568.
Article12. Cancer Genome Atlas Research Network. 2014; Integrated genomic characterization of papillary thyroid carcinoma. Cell. 159(3):676–90. DOI: 10.1016/j.cell.2014.09.050. PMID: 25417114. PMCID: PMC4243044.13. Lee J, Seol MY, Jeong S, Kwon HJ, Lee CR, Ku CR, et al. 2015; KSR1 is coordinately regulated with Notch signaling and oxidative phosphorylation in thyroid cancer. J Mol Endocrinol. 54(2):115–24. DOI: 10.1530/JME-14-0270. PMID: 25608512.
Article14. Yoshihara K, Shahmoradgoli M, Martinez E, Vegesna R, Kim H, Torres-Garcia W, et al. 2013; Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 4:2612. DOI: 10.1038/ncomms3612. PMID: 24113773. PMCID: PMC3826632.
Article15. Yuan C, Lin JZ, Sieglaff DH, Ayers SD, Denoto-Reynolds F, Baxter JD, et al. 2012; Identical gene regulation patterns of T3 and selective thyroid hormone receptor modulator GC-1. Endocrinology. 153(1):501–11. DOI: 10.1210/en.2011-1325. PMID: 22067320. PMCID: PMC3249679.
Article16. Kim KS, Min JK, Liang ZL, Lee K, Lee JU, Bae KH, et al. 2012; Aberrant l1 cell adhesion molecule affects tumor behavior and chemosensitivity in anaplastic thyroid carcinoma. Clin Cancer Res. 18(11):3071–8. DOI: 10.1158/1078-0432.CCR-11-2757. PMID: 22472175.
Article17. Semenza GL. 2012; Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med. 18(9):534–43. DOI: 10.1016/j.molmed.2012.08.001. PMID: 22921864. PMCID: PMC3449282.
Article18. Kalluri R, Weinberg RA. 2009; The basics of epithelial-mesenchymal transition. J Clin Invest. 119(6):1420–8. DOI: 10.1172/JCI39104. PMID: 19487818. PMCID: PMC2689101.
Article19. Das V, Bhattacharya S, Chikkaputtaiah C, Hazra S, Pal M. The basics of epithelial-mesenchymal transition (EMT): a study from a structure, dynamics, and functional perspective. J Cell Physiol. 2019; [Online ahead of print]. DOI: 10.1002/jcp.28160. PMID: 30723913.
Article20. Bernal J. 2005; Thyroid hormones and brain development. Vitam Horm. 71:95–122. DOI: 10.1016/S0083-6729(05)71004-9. PMID: 16112266.
Article21. Pemberton HN, Franklyn JA, Kilby MD. 2005; Thyroid hormones and fetal brain development. Minerva Ginecol. 57(4):367–78. PMID: 16170282.22. Jeong S, Kim IK, Kim H, Choi MJ, Lee J, Jo YS. 2020; Liver X receptor beta related to tumor progression and ribosome gene expression in papillary thyroid cancer. Endocrinol Metab (Seoul). 35(3):656–68. DOI: 10.3803/EnM.2020.667. PMID: 32814418. PMCID: PMC7520597.23. Lu Y, Chan YT, Tan HY, Li S, Wang N, Feng Y. 2020; Epigenetic regulation in human cancer: the potential role of epi-drug in cancer therapy. Mol Cancer. 19(1):79. DOI: 10.1186/s12943-020-01197-3. PMID: 32340605. PMCID: PMC7184703.
Article24. Zhao W, Qi X, Liu L, Ma S, Liu J, Wu J. 2020; Epigenetic regulation of m(6)A modifications in human cancer. Mol Ther Nucleic Acids. 19:405–12. DOI: 10.1016/j.omtn.2019.11.022. PMID: 31887551. PMCID: PMC6938965.
Article25. Li J, Li K, Dong X, Liang D, Zhao Q. 2014; Ncor1 and Ncor2 play essential but distinct roles in zebrafish primitive myelopoiesis. Dev Dyn. 243(12):1544–53. DOI: 10.1002/dvdy.24181. PMID: 25156564.
Article26. Hanahan D, Weinberg RA. 2011; Hallmarks of cancer: the next generation. Cell. 144(5):646–74. DOI: 10.1016/j.cell.2011.02.013. PMID: 21376230.
Article27. Choi JB, Lee WK, Lee SG, Ryu H, Lee CR, Kang SW, et al. 2018; Long-term oncologic outcomes of papillary thyroid microcarcinoma according to the presence of clinically apparent lymph node metastasis: a large retrospective analysis of 5,348 patients. Cancer Manag Res. 10:2883–91. DOI: 10.2147/CMAR.S173853. PMID: 30214283. PMCID: PMC6118257.
Article28. Lee SG, Lee WK, Lee HS, Moon J, Lee CR, Kang SW, et al. 2017; Practical performance of the 2015 American Thyroid Association guidelines for predicting tumor recurrence in patients with papillary thyroid cancer in South Korea. Thyroid. 27(2):174–81. DOI: 10.1089/thy.2016.0252. PMID: 27750028.
Article29. McKelvey BA, Zeiger MA, Umbricht CB. 2020; Exploring the epigenetic regulation of telomerase reverse transcriptase (TERT) in human cancer cell lines. Mol Oncol. 14(10):2355–7. DOI: 10.1002/1878-0261.12798. PMID: 32920953. PMCID: PMC7530778.
Article30. Yoon J, Lee E, Koo JS, Yoon JH, Nam KH, Lee J, et al. 2020; Artificial intelligence to predict the BRAFV600E mutation in patients with thyroid cancer. PLoS One. 15(11):e0242806. DOI: 10.1371/journal.pone.0242806. PMID: 33237975. PMCID: PMC7688114.
Article31. Lee WK, Lee J, Kim H, Lee SG, Choi SH, Jeong S, et al. 2019; Peripheral location and infiltrative margin predict invasive features of papillary thyroid microcarcinoma. Eur J Endocrinol. 181(2):139–49. DOI: 10.1530/EJE-18-1025. PMID: 31146263.
Article32. Lu C, Cheng SY. 2010; Thyroid hormone receptors regulate adipogenesis and carcinogenesis via crosstalk signaling with peroxisome proliferator-activated receptors. J Mol Endocrinol. 44(3):143–54. DOI: 10.1677/JME-09-0107. PMID: 19741045. PMCID: PMC3464095.
Article33. Aninye IO, Matsumoto S, Sidhaye AR, Wondisford FE. 2014; Circadian regulation of Tshb gene expression by Rev-Erbalpha (NR1D1) and nuclear corepressor 1 (NCOR1). J Biol Chem. 289(24):17070–7. DOI: 10.1074/jbc.M114.569723. PMID: 24794873. PMCID: PMC4059148.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Notch1 Signaling in Anaplastic Thyroid Carcinoma
- A case of acromegaly with mixed papillary-follicular thyroid carcinoma
- Resistance to thyroid hormone due to a novel mutation of thyroid hormone receptor beta gene
- Letter: Expression of Glucagon-Like Peptide-1 Receptor in Papillary Thyroid Carcinoma and Its Clinicopathologic Significance (Endocrinol Metab 2014;29:536-44, Min Jung Jung et al.)
- Ultrasound Findings of Diffuse Sclerosing Subtype of Papillary Thyroid Carcinoma