Arch Hand Microsurg.
2021 Dec;26(4):276-284. 10.12790/ahm.21.0101.
Six-Month Follow-up for Investigating the Effect of Prophylactic Lymphovenous Anastomosis on the Prevention of Breast Cancer-Related Lymphedema: A Preliminary Study in a Single Institution
- Affiliations
-
- 1Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- 2Department of Rehabilitation Medicine, Pusan National University School of Medicine, Busan, Korea
- 3Department of Plastic and Reconstructive Surgery, Pusan National University School of Medicine, Busan, Korea
- 4Department of Plastic and Reconstructive Surgery, Pusan National University School of Medicine, Yangsan, Korea
- KMID: 2522626
- DOI: http://doi.org/10.12790/ahm.21.0101
Abstract
- Purpose
This study was performed to assess the effect of prophylactic lymphovenous anastomosis on the prevention of arm lymphedema after axillary lymph node dissection for breast cancer treatment.
Methods
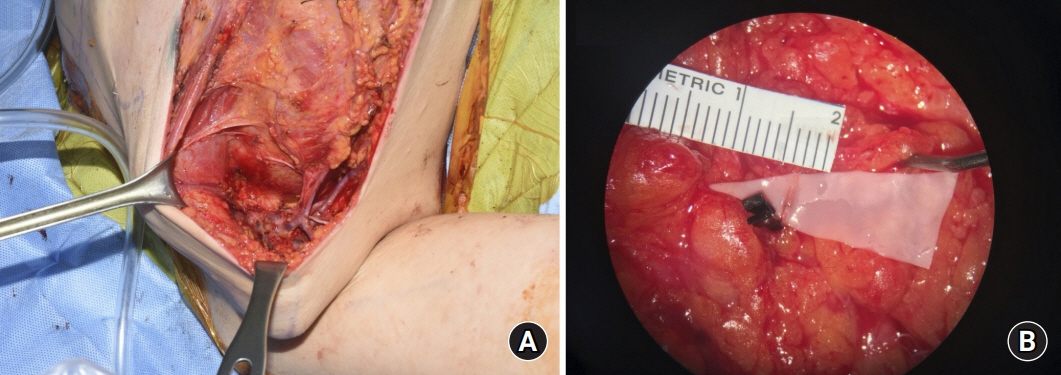
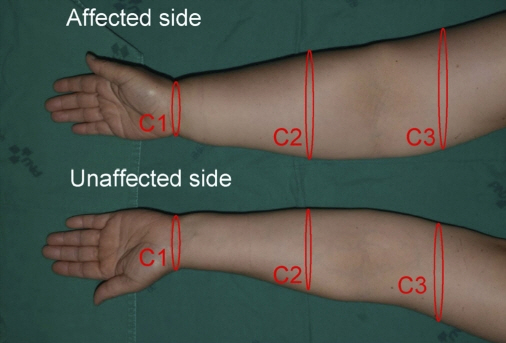
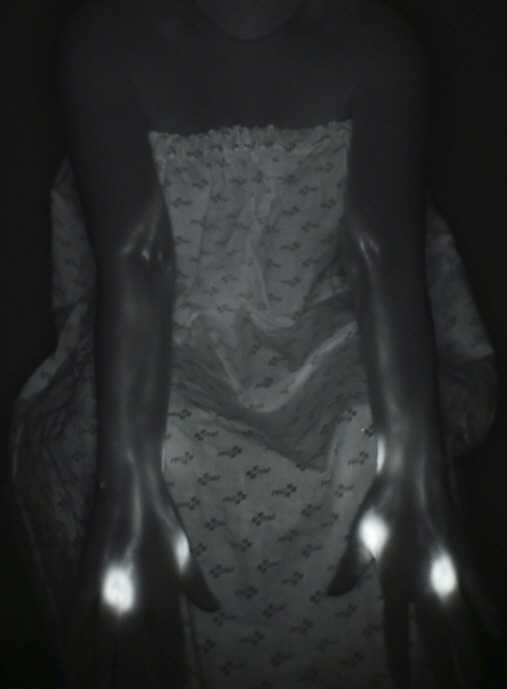
Among 69 women referred to undergo axillary lymph node dissection from January 2020 to June 2020, 21 were assigned to the treatment group and 48 to the control group. In the treatment group, 21 patients underwent prophylactic lymphovenous anastomosis for the prevention of breast cancer-related lymphedema. The other 48 patients in the control group did not undergo any preventive surgical treatment. Prophylactic lymphovenous anastomosis was performed at the same time as axillary lymph node dissection and breast cancer surgery. Postoperatively, all patients underwent circumferential measurements at 1, 3, and 6 months and lymphography at 6 months after the surgery.
Results
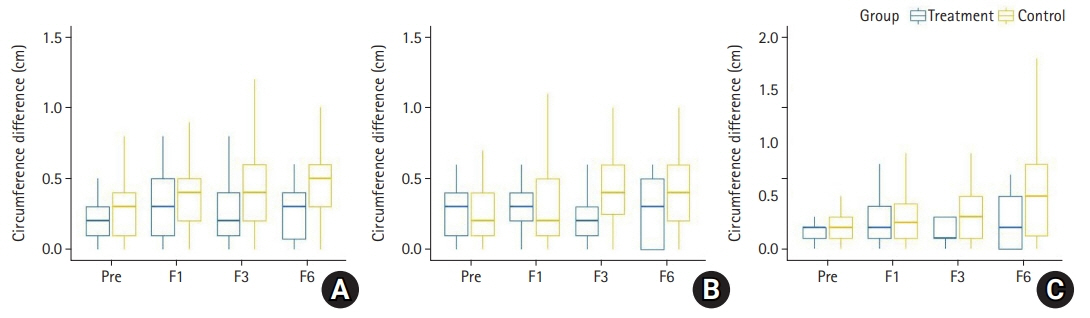
None of the patients in the treatment group had lymphedema after the surgery (0%). In the control group, lymphedema occurred in nine patients (18.8%, p=0.049). No significant differences in the arm circumference were observed in the treatment group during follow-up (p>0.05), whereas the arm circumference in the control group showed a significant increase at 1, 3, and 6 months after axillary lymph node dissection (p<0.05). There were no significant differences between the two groups in the arm circumference changes with respect to baseline at 1, 3, and 6 months after axillary lymph node dissection (p>0.05).
Conclusion
Prophylactic lymphovenous anastomosis represents a valid super microsurgical technique for the primary prevention of breast cancer-related lymphedema.
Figure
Reference
-
1. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013; 14:500–15.
Article2. Carl HM, Walia G, Bello R, et al. Systematic review of the surgical treatment of extremity lymphedema. J Reconstr Microsurg. 2017; 33:412–25.
Article3. Nguyen TT, Hoskin TL, Habermann EB, Cheville AL, Boughey JC. Breast cancer-related lymphedema risk is related to multidisciplinary treatment and not surgery alone: results from a large cohort study. Ann Surg Oncol. 2017; 24:2972–80.
Article4. McDuff SG, Mina AI, Brunelle CL, et al. Timing of lymphedema after treatment for breast cancer: when are patients most at risk? Int J Radiat Oncol Biol Phys. 2019; 103:62–70.
Article5. Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer. 2001; 92:1368–77.
Article6. Rogan S, Taeymans J, Luginbuehl H, Aebi M, Mahnig S, Gebruers N. Therapy modalities to reduce lymphoedema in female breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. 2016; 159:1–14.
Article7. Hespe GE, Nores GG, Huang JJ, Mehrara BJ. Pathophysiology of lymphedema: is there a chance for medication treatment? J Surg Oncol. 2017; 115:96–8.8. Rasmussen JC, Tan IC, Marshall MV, et al. Human lymphatic architecture and dynamic transport imaged using near-infrared fluorescence. Transl Oncol. 2010; 3:362–72.
Article9. Zhu W, Li D, Li X, et al. Association between adjuvant docetaxel-based chemotherapy and breast cancer-related lymphedema. Anticancer Drugs. 2017; 28:350–5.
Article10. Hugenholtz-Wamsteker W, Robbeson C, Nijs J, Hoelen W, Meeus M. The effect of docetaxel on developing oedema in patients with breast cancer: a systematic review. Eur J Cancer Care (Engl). 2016; 25:269–79.
Article11. Kim M, Kim SW, Lee SU, et al. A model to estimate the risk of breast cancer-related lymphedema: combinations of treatment-related factors of the number of dissected axillary nodes, adjuvant chemotherapy, and radiation therapy. Int J Radiat Oncol Biol Phys. 2013; 86:498–503.
Article12. Sener SF, Winchester DJ, Martz CH, et al. Lymphedema after sentinel lymphadenectomy for breast carcinoma. Cancer. 2001; 92:748–52.
Article13. Wilke LG, McCall LM, Posther KE, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol. 2006; 13:491–500.
Article14. Thompson M, Korourian S, Henry-Tillman R, et al. Axillary reverse mapping (ARM): a new concept to identify and enhance lymphatic preservation. Ann Surg Oncol. 2007; 14:1890–5.
Article15. Nos C, Lesieur B, Clough KB, Lecuru F. Blue dye injection in the arm in order to conserve the lymphatic drainage of the arm in breast cancer patients requiring an axillary dissection. Ann Surg Oncol. 2007; 14:2490–6.
Article16. Boccardo FM, Casabona F, Friedman D, et al. Surgical prevention of arm lymphedema after breast cancer treatment. Ann Surg Oncol. 2011; 18:2500–5.
Article17. Demirtas Y, Ozturk N, Yapici O, Topalan M. Supermicrosurgical lymphaticovenular anastomosis and lymphaticovenous implantation for treatment of unilateral lower extremity lymphedema. Microsurgery. 2009; 29:609–18.
Article18. Tiwari P, Coriddi M, Salani R, Povoski SP. Breast and gynecologic cancer-related extremity lymphedema: a review of diagnostic modalities and management options. World J Surg Oncol. 2013; 11:237.
Article19. Ishiura R, Yamamoto T, Saito T, Mito D, Iida T. Comparison of lymphovenous shunt methods in a rat model: supermicrosurgical lymphaticovenular anastomosis versus microsurgical lymphaticovenous implantation. Plast Reconstr Surg. 2017; 139:1407–13.20. Yamamoto T, Yamamoto N, Yamashita M, Furuya M, Hayashi A, Koshima I. Efferent lymphatic vessel anastomosis: supermicrosurgical efferent lymphatic vessel-to-venous anastomosis for the prophylactic treatment of subclinical lymphedema. Ann Plast Surg. 2016; 76:424–7.21. Takeishi M, Kojima M, Mori K, Kurihara K, Sasaki H. Primary intrapelvic lymphaticovenular anastomosis following lymph node dissection. Ann Plast Surg. 2006; 57:300–4.
Article22. Armer JM, Stewart BR, Smith K, Cormier JN. Lymphedema following cancer treatment. In : Lester JL, Schmitt PA, editors. Cancer rehabilitation and survivorship: transdisciplinary approaches to personalized care. Pittsburgh, PA: Oncology Nursing Society;2011. p. 133–43.23. Kilbreath SL, Lee MJ, Refshauge KM, et al. Transient swelling versus lymphoedema in the first year following surgery for breast cancer. Support Care Cancer. 2013; 21:2207–15.
Article24. Johnson AR, Fleishman A, Granoff MD, et al. Evaluating the impact of immediate lymphatic reconstruction for the surgical prevention of lymphedema. Plast Reconstr Surg. 2021; 147:373e–381e.
Article25. Boccardo F, Casabona F, De Cian F, et al. Lymphatic microsurgical preventing healing approach (LYMPHA) for primary surgical prevention of breast cancer-related lymphedema: over 4 years follow-up. Microsurgery. 2014; 34:421–4.
Article26. Feldman S, Bansil H, Ascherman J, et al. Single institution experience with lymphatic microsurgical preventive healing approach (LYMPHA) for the primary prevention of lymphedema. Ann Surg Oncol. 2015; 22:3296–301.
Article27. Casabona F, Bogliolo S, Valenzano Menada M, Sala P, Villa G, Ferrero S. Feasibility of axillary reverse mapping during sentinel lymph node biopsy in breast cancer patients. Ann Surg Oncol. 2009; 16:2459–63.
Article28. Onoda S, Todokoro T, Hara H, Azuma S, Goto A. Minimally invasive multiple lymphaticovenular anastomosis at the ankle for the prevention of lower leg lymphedema. Microsurgery. 2014; 34:372–6.
Article29. Gärtner R, Jensen MB, Kronborg L, Ewertz M, Kehlet H, Kroman N. Self-reported arm-lymphedema and functional impairment after breast cancer treatment: a nationwide study of prevalence and associated factors. Breast. 2010; 19:506–15.30. Ashikaga T, Krag DN, Land SR, et al. Morbidity results from the NSABP B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol. 2010; 102:111–8.
Article31. Wernicke AG, Goodman RL, Turner BC, et al. A 10-year follow-up of treatment outcomes in patients with early stage breast cancer and clinically negative axillary nodes treated with tangential breast irradiation following sentinel lymph node dissection or axillary clearance. Breast Cancer Res Treat. 2011; 125:893–902.
Article32. Zou L, Liu FH, Shen PP, et al. The incidence and risk factors of related lymphedema for breast cancer survivors post-operation: a 2-year follow-up prospective cohort study. Breast Cancer. 2018; 25:309–14.
Article33. Heineman JT, Chen WF. Indocyaine green lymphography to diagnose primary lymphedema and the incidental discovery of primary asymptomatic lymphatic insufficiency. Plast Reconstr Surg Glob Open. 2018; 6(9 Suppl):55–6.34. Johansson K, Branje E. Arm lymphoedema in a cohort of breast cancer survivors 10 years after diagnosis. Acta Oncol. 2010; 49:166–73.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevention of Breast Cancer-Related Lymphedema Using the Lymphatic Microsurgical Preventive Healing Approach: A Case Report
- Efficacy and patient satisfaction regarding lymphovenous bypass with sleeve-in anastomosis for extremity lymphedema
- Persistent retrograde venous-lymphatic reflux in side-to-end lymphaticovenous anastomosis in a lower extremity with lymphedema: a case report
- Breast cancer related lymphedema and surgical treatment
- Effects of Lymphovenous Anastomosis Surgery Using Ultrasonography in Lymphedema From a Pressure Perspective