Korean Circ J.
2021 Nov;51(11):899-907. 10.4070/kcj.2021.0239.
Role of Genetics in Preventive Cardiology: Focused on Dyslipidemia
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2522107
- DOI: http://doi.org/10.4070/kcj.2021.0239
Abstract
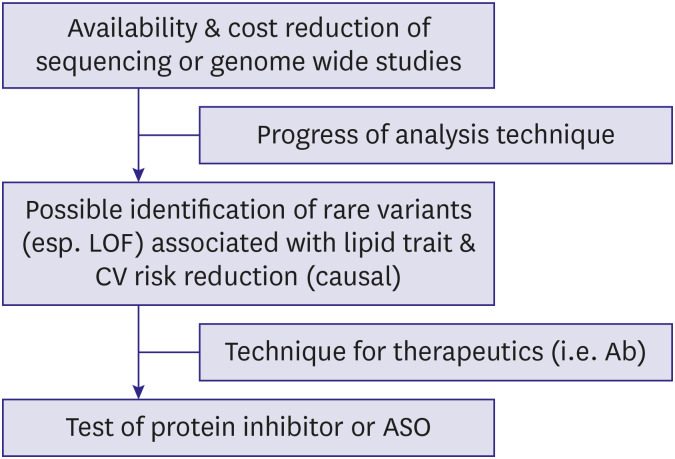
- Dyslipidemia is a strong risk factor for cardiovascular disease as well as a major target for its prevention. Along with the progress in genetic research techniques and bioinformatics analysis, genetic knowledge helps manage individuals with dyslipidemia. Familial hypercholesterolemia, the most common monogenic lipid disorder, can be diagnosed clinically without confirming pathogenic mutations. However, it can be difficult to do so due to uncertain family history, and genetic testing is of vital importance in such cases. Conversely, recent studies have revealed that combination effect of rare and common variants is frequent in people with other extreme lipid phenotypes. Genetic characteristics are helpful for prediction and selection of patients with high risk for cardiovascular disease or poor response to lipid-lowering therapy. In the past decade, studies using new genetic techniques have identified novel associations among lipid metabolism-associated genes, intermediate lipid phenotypes, and cardiovascular health. Such findings shed light on new drug targets. With improvements in the platforms and processes for drug development, several recent clinical trials showed promising results regarding lipid control and potential cardiovascular disease prevention.
Figure
Reference
-
1. Koo BK, Park S, Han KD, Moon MK. Hypertriglyceridemia is an independent risk factor for cardiovascular diseases in Korean adults aged 30–49 years: a nationwide population-based study. J Lipid Atheroscler. 2021; 10:88–98. PMID: 33537256.
Article2. Cho SM, Lee H, Lee HH, et al. Dyslipidemia fact sheets in Korea 2020: an analysis of nationwide population-based data. J Lipid Atheroscler. 2021; 10:202–209. PMID: 34095012.
Article3. McPherson R, Tybjaerg-Hansen A. Genetics of coronary artery disease. Circ Res. 2016; 118:564–578. PMID: 26892958.
Article4. Lee CJ, Lee JH, Choi S, et al. Screening, diagnosis, and treatment of familial hypercholesterolemia: symposium of the education committee, Korean Society of Lipid and Atherosclerosis. J Lipid Atheroscler. 2018; 7:122–154.
Article5. Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020; 41:111–188. PMID: 31504418.6. Han SM, Hwang B, Park TG, et al. Genetic testing of Korean familial hypercholesterolemia using whole-exome sequencing. PLoS One. 2015; 10:e0126706. PMID: 25962062.
Article7. Yamashita S, Sprecher DL, Sakai N, Matsuzawa Y, Tarui S, Hui DY. Accumulation of apolipoprotein E-rich high density lipoproteins in hyperalphalipoproteinemic human subjects with plasma cholesteryl ester transfer protein deficiency. J Clin Invest. 1990; 86:688–695. PMID: 2118552.
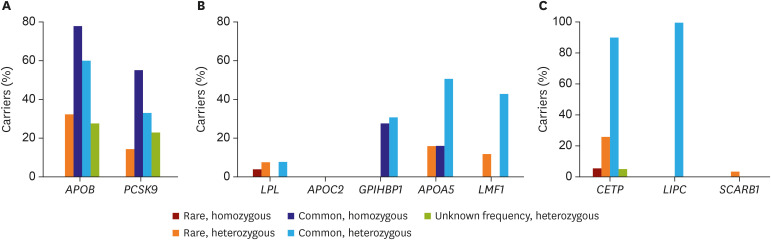
Article8. Lee CJ, Lee Y, Park S, et al. Rare and common variants of APOB and PCSK9 in Korean patients with extremely low low-density lipoprotein-cholesterol levels. PLoS One. 2017; 12:e0186446. PMID: 29036232.
Article9. Lee CJ, Oum CY, Lee Y, et al. Variants of lipolysis-related genes in Korean patients with very high triglycerides. Yonsei Med J. 2018; 59:148–153. PMID: 29214790.
Article10. Lee CJ, Park MS, Kim M, et al. CETP, LIPC, and SCARB1 variants in individuals with extremely high high-density lipoprotein-cholesterol levels. Sci Rep. 2019; 9:10915. PMID: 31358896.
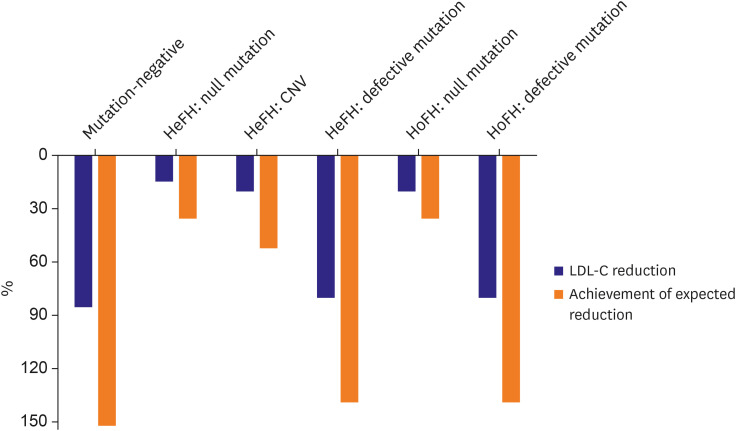
Article11. Tada H, Kawashiri MA, Nohara A, Inazu A, Mabuchi H, Yamagishi M. Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur Heart J. 2017; 38:1573–1579. PMID: 28159968.12. Santos PC, Morgan AC, Jannes CE, et al. Presence and type of low density lipoprotein receptor (LDLR) mutation influences the lipid profile and response to lipid-lowering therapy in Brazilian patients with heterozygous familial hypercholesterolemia. Atherosclerosis. 2014; 233:206–210. PMID: 24529145.
Article13. Santos PC, Pereira AC. Type of LDLR mutation and the pharmacogenetics of familial hypercholesterolemia treatment. Pharmacogenomics. 2015; 16:1743–1750. PMID: 26427613.
Article14. Perez de Isla L, Alonso R, Watts GF, et al. Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia: 5-year SAFEHEART registry follow-up. J Am Coll Cardiol. 2016; 67:1278–1285. PMID: 26988947.15. Kim H, Lee CJ, Pak H, et al. GENetic characteristics and REsponse to lipid-lowering therapy in familial hypercholesterolemia: GENRE-FH study. Sci Rep. 2020; 10:19336. PMID: 33168860.
Article16. Postmus I, Trompet S, Deshmukh HA, et al. Pharmacogenetic meta-analysis of genome-wide association studies of LDL cholesterol response to statins. Nat Commun. 2014; 5:5068. PMID: 25350695.17. Natarajan P, Young R, Stitziel NO, et al. Polygenic risk score identifies subgroup with higher burden of atherosclerosis and greater relative benefit from statin therapy in the primary prevention setting. Circulation. 2017; 135:2091–2101. PMID: 28223407.
Article18. Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010; 466:707–713. PMID: 20686565.19. Crosby J, Peloso GM, Auer PL, et al. Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med. 2014; 371:22–31. PMID: 24941081.
Article20. Spiller W, Jung KJ, Lee JY, Jee SH. Precision medicine and cardiovascular health: insights from Mendelian randomization analyses. Korean Circ J. 2020; 50:91–111. PMID: 31845553.
Article21. Lee SH, Lee JY, Kim GH, et al. Two-sample Mendelian randomization study of lipid levels and ischemic heart disease. Korean Circ J. 2020; 50:940–948. PMID: 32812402.
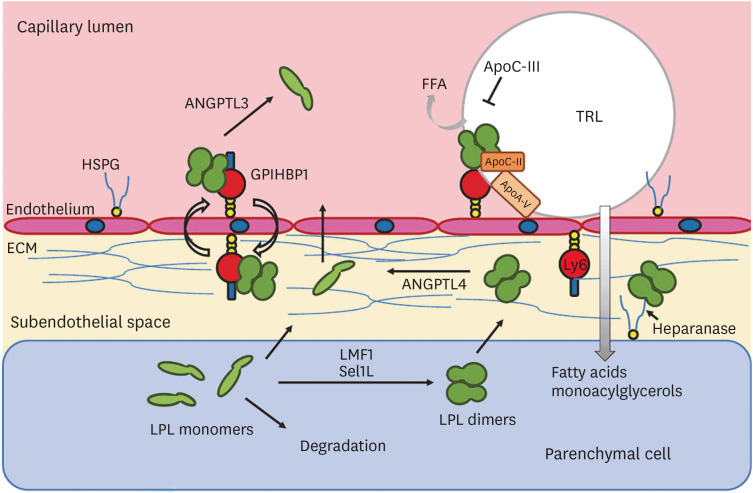
Article22. Olivecrona G. Role of lipoprotein lipase in lipid metabolism. Curr Opin Lipidol. 2016; 27:233–241. PMID: 27031275.
Article23. Musunuru K, Pirruccello JP, Do R, et al. Exome sequencing, ANGPTL3 mutations, and familial combined hypolipidemia. N Engl J Med. 2010; 363:2220–2227. PMID: 20942659.24. Dewey FE, Gusarova V, O'Dushlaine C, et al. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med. 2016; 374:1123–1133. PMID: 26933753.
Article25. Levin AA. Treating disease at the RNA level with oligonucleotides. N Engl J Med. 2019; 380:57–70. PMID: 30601736.
Article26. Raal FJ, Rosenson RS, Reeskamp LF, et al. Evinacumab for homozygous familial hyperchoelsterolemia. N Engl J Med. 2020; 383:711–720. PMID: 32813947.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New European Society of Cardiology/European Atherosclerosis Society Guideline for the Management of Dyslipidemia
- Epidemiology of dyslipidemia in Korea
- Prevalence of Dyslipidemia among Korean Adults: Korea National Health and Nutrition Survey 1998-2005
- Recent Guideline for the Management of Dyslipidemia in Patients with Diabetes
- Recent dyslipidemia guidelines for patients with diabetes mellitus