J Korean Neurosurg Soc.
2021 Nov;64(6):983-994. 10.3340/jkns.2021.0165.
The Tumor Control According to Radiation Dose of Gamma Knife Radiosurgery for Small and Medium-Sized Brain Metastases from Non-Small Cell Lung Cancer
- Affiliations
-
- 1Department of Neurosurgery, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, Korea
- 2Department of Radiology, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, Korea
- 3Department of Internal Medicine, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, Korea
- KMID: 2521989
- DOI: http://doi.org/10.3340/jkns.2021.0165
Abstract
Objective
: The effectiveness of gamma knife radiosurgery (GKR) in the treatment of brain metastases is well established. The aim of this study was to evaluate the efficacy and safety of maximizing the radiation dose in GKR and the factors influencing tumor control in cases of small and medium-sized brain metastases from non-small cell lung cancer (NSCLC).
Methods
: We analyzed 230 metastatic brain tumors less than 5 mL in volume in 146 patients with NSCLC who underwent GKR. The patients had no previous radiation therapy for brain metastases. The pathologies of the tumors were adenocarcinoma (n=207), squamous cell carcinoma (n=18), and others (n=5). The radiation doses were classified as 18, 20, 22, and 24 Gy, and based on the tumor volume, the tumors were categorized as follows : small-sized (less than 1 mL) and medium-sized (1–3 and 3–5 mL). The progression-free survival (PFS) of the individual 230 tumors and 146 brain metastases was evaluated after GKR depending on the pathology, Eastern Cooperative Oncology Group (ECOG) performance score (PS), tumor volume, radiation dose, and anti-cancer regimens. The radiotoxicity after GKR was also evaluated.
Results
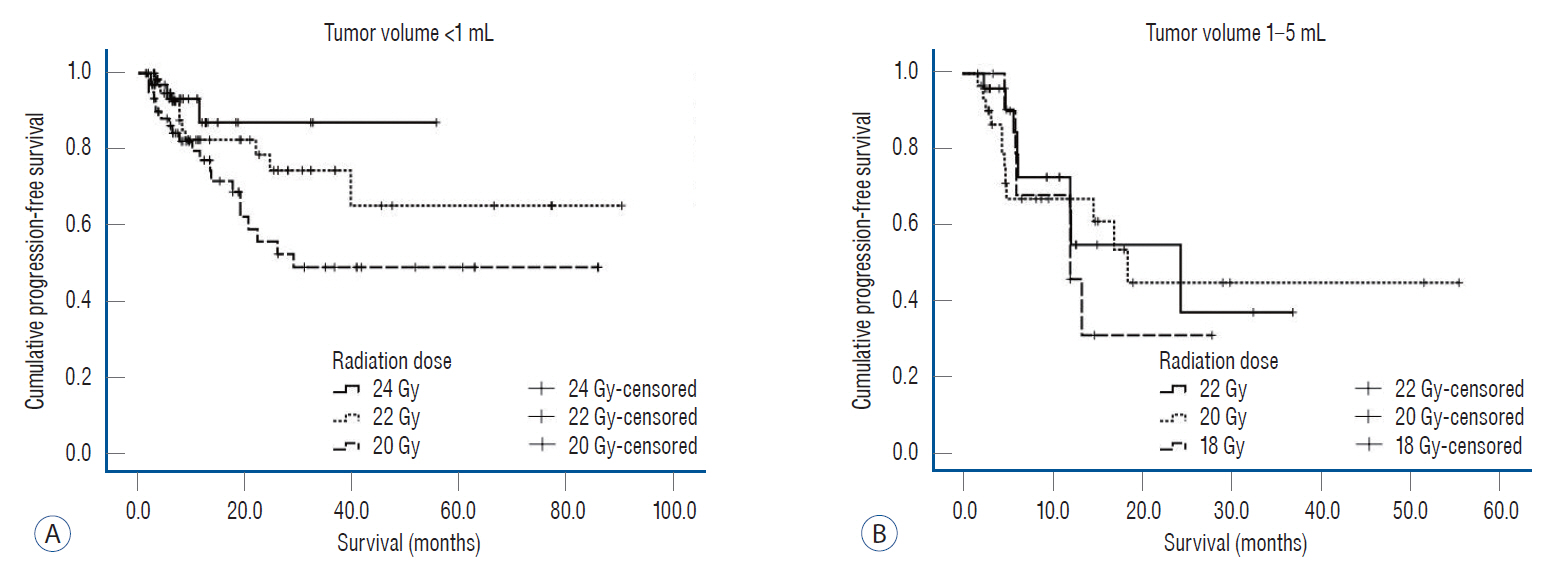
: After GKR, the restricted mean PFS of individual 230 tumors at 24 months was 15.6 months (14.0–17.1). In small-sized tumors, as the dose of radiation increased, the tumor control rates tended to increase (p=0.072). In medium-sized tumors, there was no statistically difference in PFS with an increase of radiation dose (p=0.783). On univariate analyses, a statistically significant increase in PFS was associated with adenocarcinomas (p=0.001), tumors with ECOG PS 0 (p=0.005), small-sized tumors (p=0.003), radiation dose of 24 Gy (p=0.014), synchronous lesions (p=0.002), and targeted therapy (p=0.004). On multivariate analyses, an improved PFS was seen with targeted therapy (hazard ratio, 0.356; 95% confidence interval, 0.150–0.842; p=0.019). After GKR, the restricted mean PFS of brain at 24 months was 9.8 months (8.5–11.1) in 146 patients, and the pattern of recurrence was mostly distant within the brain (66.4%). The small and medium-sized tumors treated with GKR showed radiotoxicitiy in five out of 230 tumors (2.2%), which were controlled with medical treatment.
Conclusion
: The small-sized tumors were effectively controlled without symptomatic radiation necrosis as the radiation dose was increased up to 24 Gy. The medium-sized tumors showed potential for symptomatic radiation necrosis without signifcant tumor control rate, when greater than 18 Gy. GKR combined targeted therapy improved the tumor control of GKR-treated tumors.
Figure
Cited by 1 articles
-
The Optimal Time between Embolization and Surgery for Hypervascular Spinal Metastatic Tumors : A Systematic Review and Meta-Analysis
Woon Tak Yuh, Junghoon Han, Chang-Hyun Lee, Chi Heon Kim, Hyun-Seung Kang, Chun Kee Chung
J Korean Neurosurg Soc. 2023;66(4):438-445. doi: 10.3340/jkns.2022.0204.
Reference
-
References
1. Abdallah SM, Wong A. Brain metastases in non-small-cell lung cancer: are tyrosine kinase inhibitors and checkpoint inhibitors now viable options? Curr Oncol. 25(Suppl 1):S103–S114. 2018.
Article2. Baschnagel AM, Meyer KD, Chen PY, Krauss DJ, Olson RE, Pieper DR, et al. Tumor volume as a predictor of survival and local control in patients with brain metastases treated with gamma knife surgery. J Neurosurg. 119:1139–1144. 2013.
Article3. Blagden SP, Charman SC, Sharples LD, Magee LR, Gilligan D. Performance status score: do patients and their oncologists agree? Br J Cancer. 89:1022–1027. 2003.
Article4. Broniscer A, Panetta JC, O'Shaughnessy M, Fraga C, Bai F, Krasin MJ, et al. Plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite OSI-420. Clin Cancer Res. 13:1511–1515. 2007.
Article5. Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 4:36–54. 2015.6. Chin LS, Ma L, DiBiase S. Radiation necrosis following gamma knife surgery: a case-controlled comparison of treatment parameters and long-term clinical follow up. J Neurosurg. 94:899–904. 2001.
Article7. Fan Y, Huang Z, Fang L, Miu L, Lin N, Gong L, et al. Chemotherapy and EGFR tyrosine kinase inhibitors for treatment of brain metastases from non-small-cell lung cancer: survival analysis in 210 patients. Onco Targets Ther. 6:1789–1803. 2013.8. Frischer JM, Fraller A, Mallouhi A, Vogl UM, Baier F, Ertl A, et al. Evaluation of dose-staged gamma knife radiosurgical treatment method for high-risk brain metastases. World Neurosurg. 94:352–359. 2016.
Article9. Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 37:745–751. 1997.
Article10. Higuchi Y, Serizawa T, Nagano O, Matsuda S, Ono J, Sato M, et al. Three-staged stereotactic radiotherapy without whole brain irradiation for large metastatic brain tumors. Int J Radiat Oncol Biol Phys. 74:1543–1548. 2009.
Article11. Hochmair M. Medical treatment options for patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer suffering from brain metastases and/or leptomeningeal disease. Target Oncol. 13:269–285. 2018.
Article12. Jung EW, Choi J, Chao ST, Murphy ES, Suh JH. Chapter 8 - Principles and Tenets of Radiation Treatment in Glioblastoma. In : Brem S, Abdullah KG, editors. Glioblastoma. ed 1. Amsterdam: Elsevier;2016. p. 105–132.13. Kamiryo T, Lopes MB, Kassell NF, Steiner L, Lee KS. Radiosurgery-induced microvascular alterations precede necrosis of the brain neuropil. Neurosurgery. 49:409–414. discussion 414-415. 2001.
Article14. Kim KH, Lee MH, Cho KR, Choi JW, Kong DS, Seol HJ, et al. The influence of histology on the response of brain metastases to gamma knife radiosurgery: a propensity score-matched study. Acta Neurochir (Wien). 160:2379–2386. 2018.
Article15. Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 16:e270–e278. 2015.
Article16. Mekhail T, Sombeck M, Sollaccio R. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. Curr Oncol Rep. 13:255–258. 2011.
Article17. Miyatake S, Nonoguchi N, Furuse M, Yoritsune E, Miyata T, Kawabata S, et al. Pathophysiology, diagnosis, and treatment of radiation necrosis in the brain. Neurol Med Chir (Tokyo). 55:50–59. 2015.
Article18. Mohammadi AM, Schroeder JL, Angelov L, Chao ST, Murphy ES, Yu JS, et al. Impact of the radiosurgery prescription dose on the local control of small (2 cm or smaller) brain metastases. J Neurosurg. 126:735–743. 2017.
Article19. Nonoguchi N, Miyatake S, Fukumoto M, Furuse M, Hiramatsu R, Kawabata S, et al. The distribution of vascular endothelial growth factor-producing cells in clinical radiation necrosis of the brain: pathological consideration of their potential roles. J Neurooncol. 105:423–431. 2011.
Article20. Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 377:829–838. 2017.
Article21. Reungwetwattana T, Nakagawa K, Cho BC, Cobo M, Cho EK, Bertolini A, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 36:33, 3290-3297. 2018.22. Schimmel WCM, Verhaak E, Hanssens PEJ, Gehring K, Sitskoorn MM. A randomised trial to compare cognitive outcome after gamma knife radiosurgery versus whole brain radiation therapy in patients with multiple brain metastases: research protocol CAR-study B. BMC Cancer. 18:218. 2018.
Article23. Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 47:291–298. 2000.
Article24. Sun YW, Xu J, Zhou J, Liu WJ. Targeted drugs for systemic therapy of lung cancer with brain metastases. Oncotarget. 9:5459–5472. 2018.
Article25. Trnovec T, Kállay Z, Bezek S. Effects of ionizing radiation on the blood brain barrier permeability to pharmacologically active substances. Int J Radiat Oncol Biol Phys. 19:1581–1587. 1990.
Article26. van Vulpen M, Kal HB, Taphoorn MJ, El-Sharouni SY. Changes in blood-brain barrier permeability induced by radiotherapy: implications for timing of chemotherapy? (Review). Oncol Rep. 9:683–688. 2002.
Article27. Vogelbaum MA, Angelov L, Lee SY, Li L, Barnett GH, Suh JH. Local control of brain metastases by stereotactic radiosurgery in relation to dose to the tumor margin. J Neurosurg. 104:907–912. 2006.
Article28. Wrona A, Dziadziuszko R, Jassem J. Management of brain metastases in non-small cell lung cancer in the era of tyrosine kinase inhibitors. Cancer Treat Rev. 71:59–67. 2018.
Article29. Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 15:387–395. 2014.
Article30. Yomo S, Hayashi M. A minimally invasive treatment option for large metastatic brain tumors: long-term results of two-session Gamma knife stereotactic radiosurgery. Radiat Oncol. 9:132. 2014.
Article31. Yu JB, Schulder M, Knisely J. Radiosurgical dose selection for brain metastasis. Prog Neurol Surg. 25:139–147. 2012.
Article32. Zhao J, Chen M, Zhong W, Zhang L, Li L, Xiao Y, et al. Cerebrospinal fluid concentrations of gefitinib in patients with lung adenocarcinoma. Clin Lung Cancer. 14:188–193. 2013.
Article33. Zhuang H, Shi S, Yuan Z, Chang JY. Bevacizumab treatment for radiation brain necrosis: mechanism, efficacy and issues. Mol Cancer. 18:21. 2019.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Analysis of Gamma Knife Radiosurgery for Brain Metastases
- Two-Day Fraction Gamma Knife Radiosurgery for Large Brain Metastasis
- Gamma Knife Radiosurgery for Vestibular Schwannomas
- Gamma Knife Radiosurgery after Stereotactic Aspiration for Large Cystic Brain Metastases
- Comparison of Stereotactic Radiosurgery and Whole Brain Radiotherapy in Patients with Four or More Brain Metastases