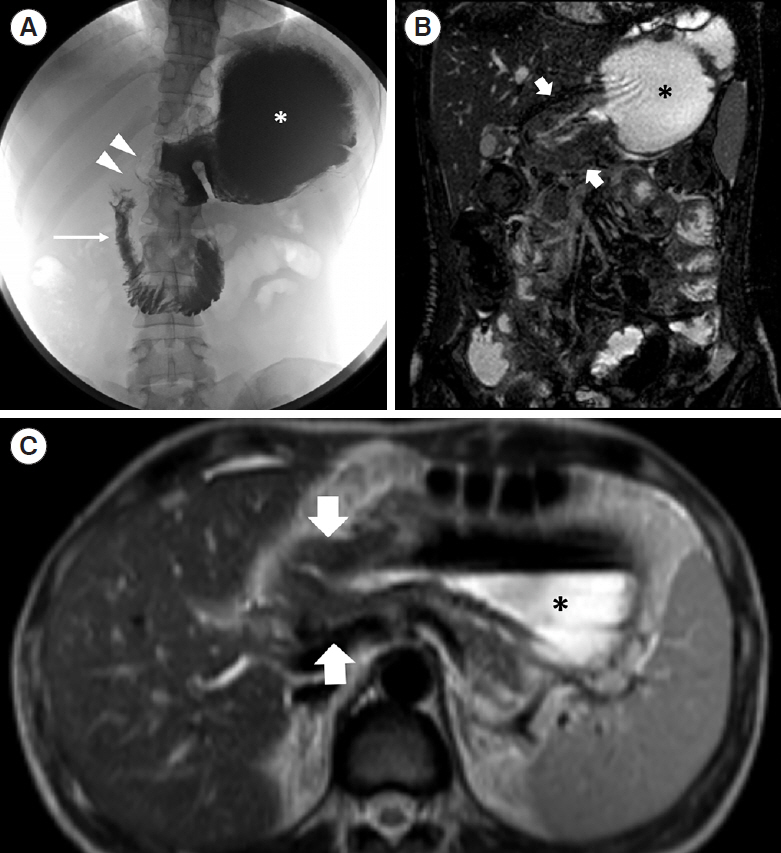
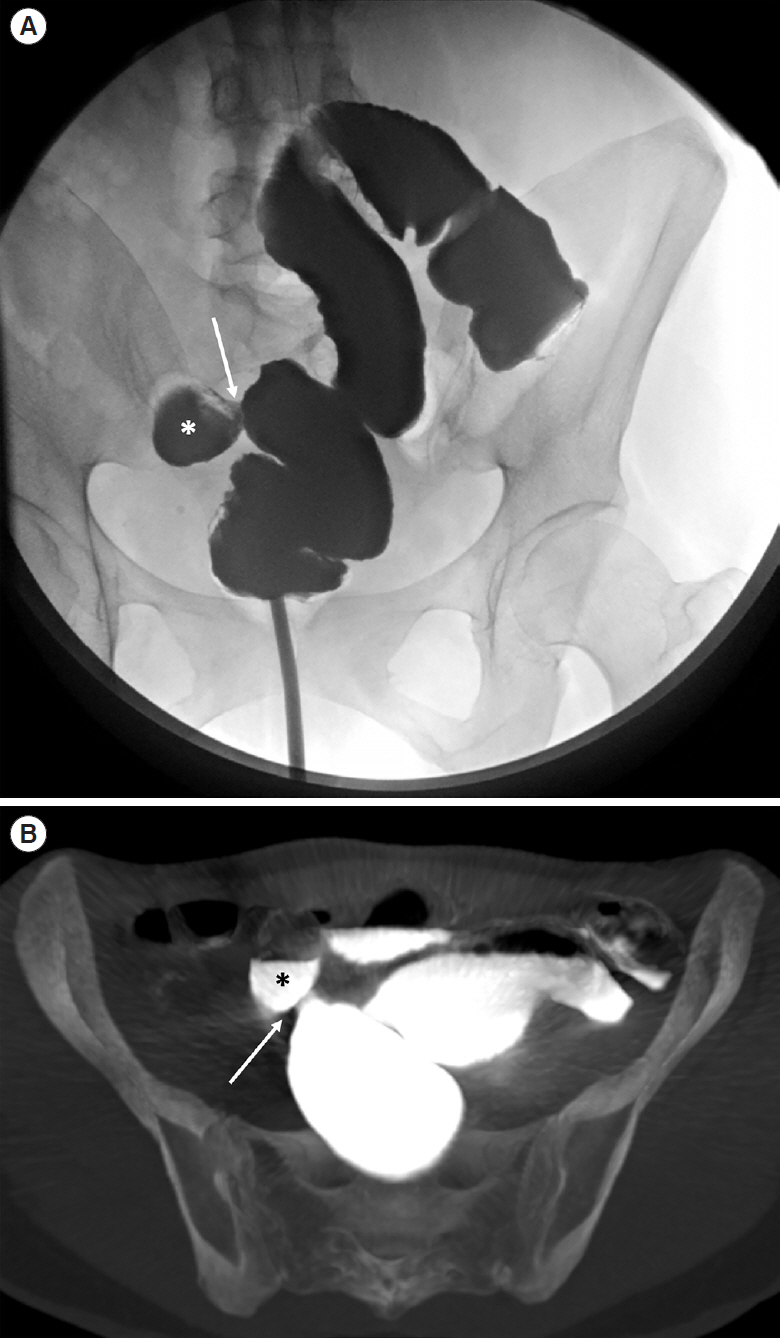
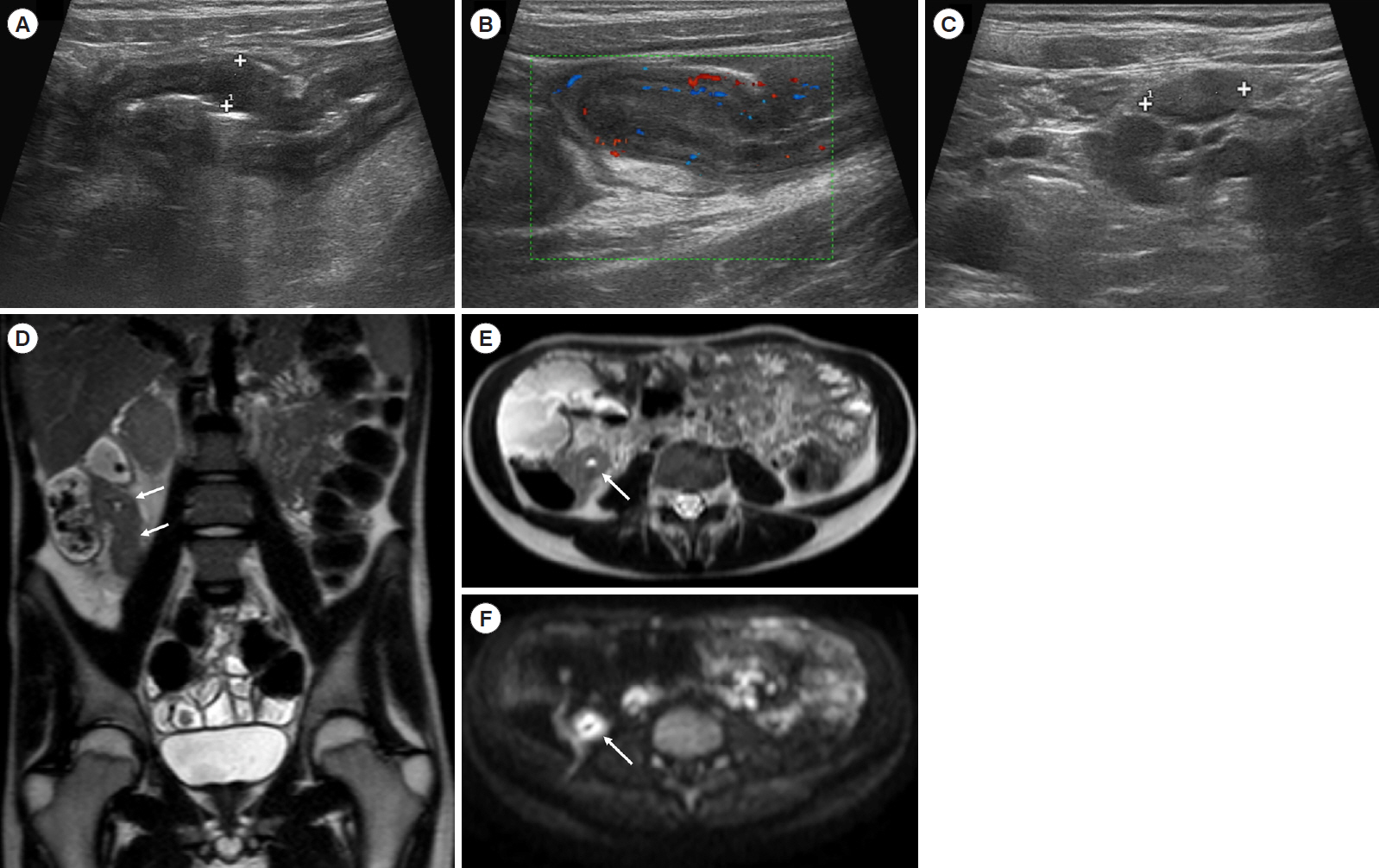
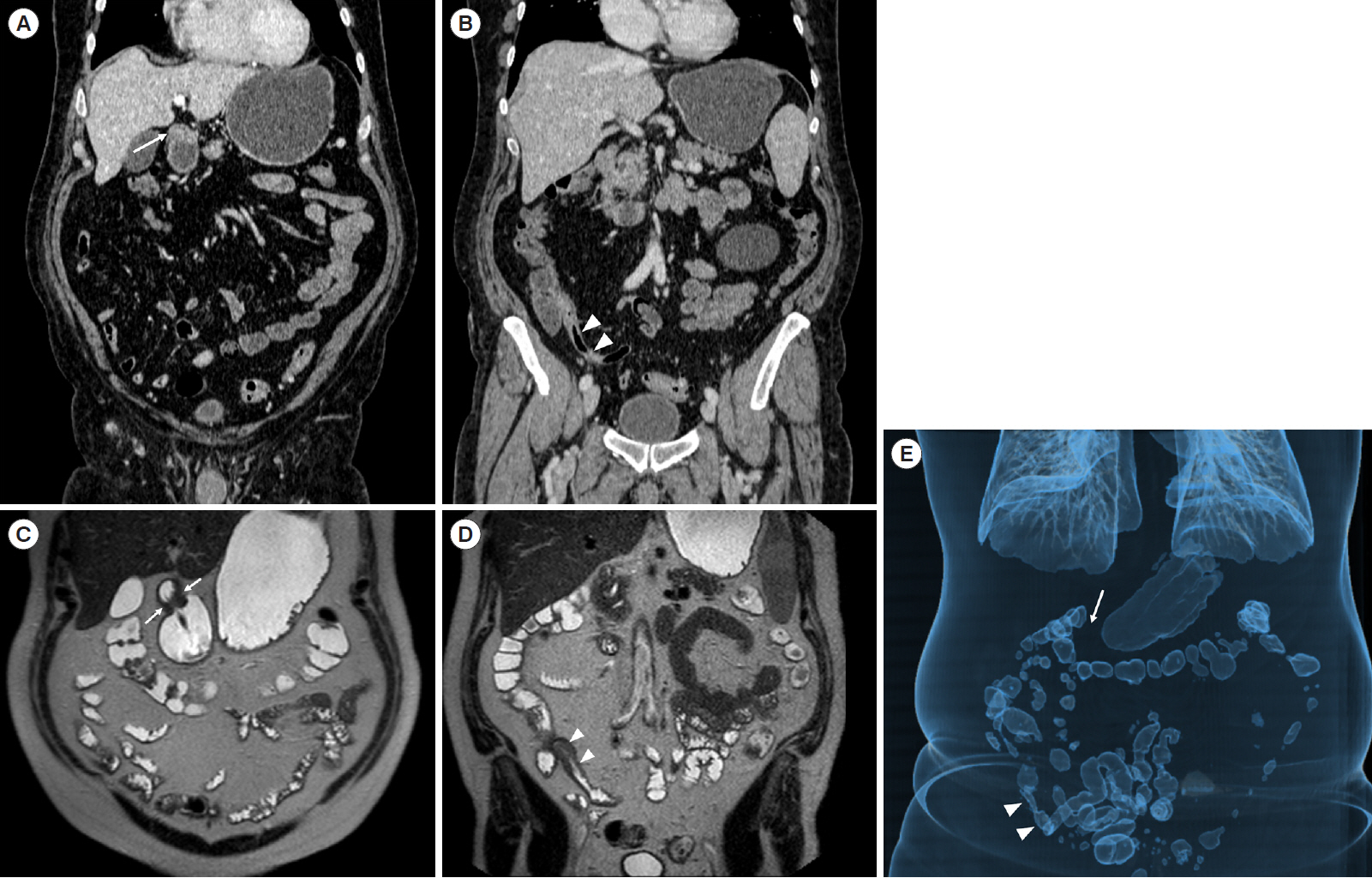
Intest Res.
2021 Oct;19(4):365-378. 10.5217/ir.2020.00097.
Crohn’s disease at radiological imaging: focus on techniques and intestinal tract
- Affiliations
-
- 1Section of Radiological Sciences, Department of Biomedical Sciences and Morphological and Functional Imaging, University of Messina, Messina, Italy
- KMID: 2521600
- DOI: http://doi.org/10.5217/ir.2020.00097
Abstract
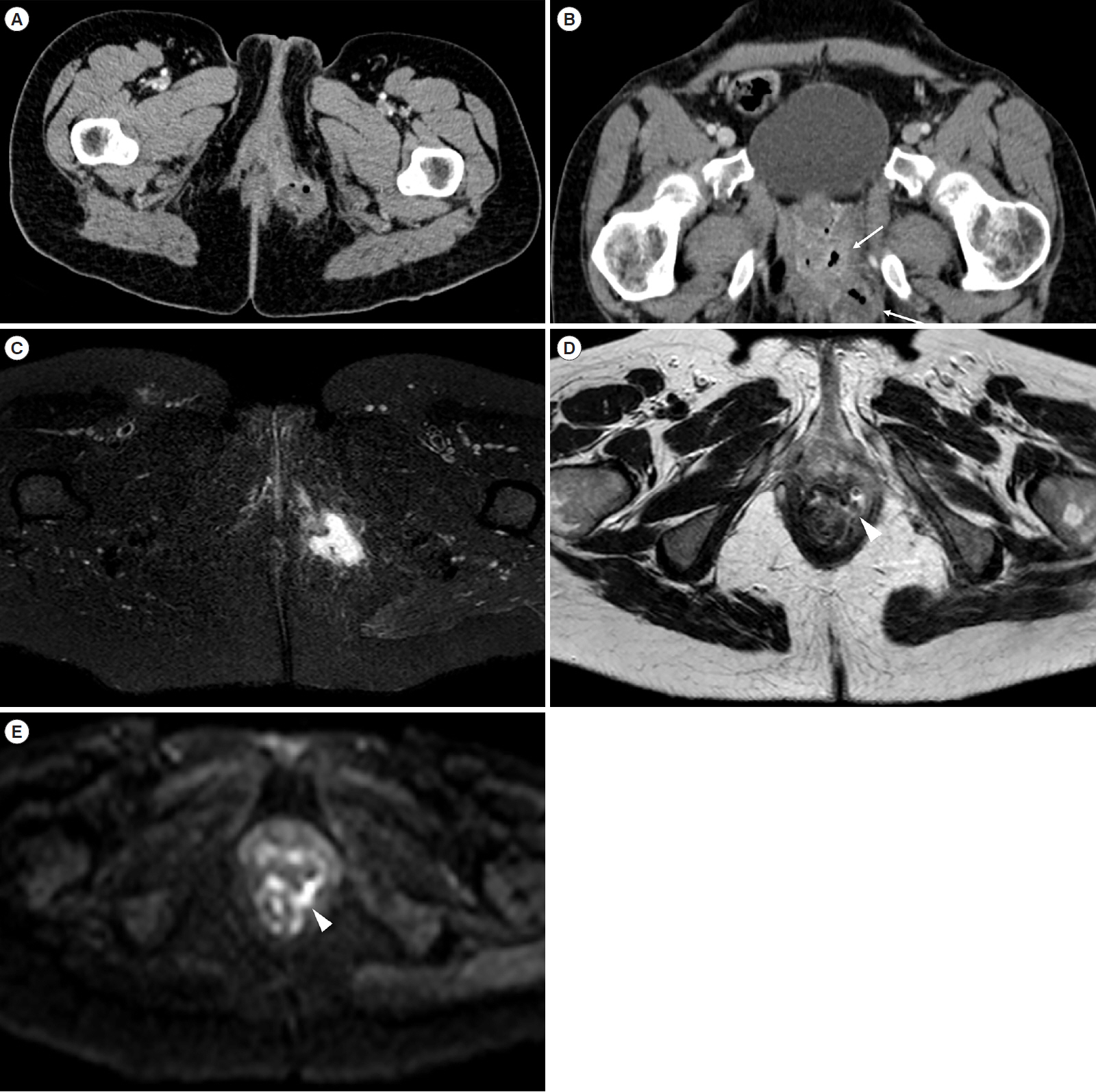
- Over recent years, inflammatory bowel diseases have become an issue of increased attention in daily clinical practice, due to both a rising incidence and improved imaging capability in detection. In particular, the diagnosis of Crohn’s disease is based on clinical picture, laboratory tests and colonoscopy with biopsy. However, colonoscopic evaluation is limited to the mucosal layer. Thus, imaging modalities play a pivotal role in enriching the clinical picture, delivering information on intestinal and extraintestinal involvement. All the imaging modalities can be employed in evaluation of Crohn’s disease patients, each of them with specific strengths as well as limitations. In this wide selection, the choice of a proper diagnostic framework can be challenging for the clinician. Therefore, the aim of this work is to offer an overview of the different imaging techniques, with brief technical details and diagnostic potential related to each intestinal tract.
Figure
Cited by 1 articles
-
Achieving high-quality magnetic resonance enterography is critical for assessing Crohn’s disease activity
Kyoung Doo Song
Intest Res. 2024;22(2):117-118. doi: 10.5217/ir.2024.0043.
Reference
-
1. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet. 2017; 389:1741–1755.
Article2. GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020; 5:17–30.3. Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018; 16:26–42.4. Kim JM, Cheon JH. Pathogenesis and clinical perspectives of extraintestinal manifestations in inflammatory bowel diseases. Intest Res. 2020; 18:249–264.5. Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011; 7:235–241.6. Pokala A, Shen B. Update of endoscopic management of Crohn’s disease strictures. Intest Res. 2020; 18:1–10.7. Hirai F. Current status of endoscopic balloon dilation for Crohn’s disease. Intest Res. 2017; 15:166–173.
Article8. Kucharzik T, Maaser C. Intestinal ultrasound and management of small bowel Crohn’s disease. Therap Adv Gastroenterol. 2018; 11:1756284818771367.
Article9. Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019; 13:144–164.
Article10. Sturm A, Maaser C, Calabrese E, et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019; 13:273–284.
Article11. Mocci G, Migaleddu V, Cabras F, et al. SICUS and CEUS imaging in Crohn’s disease: an update. J Ultrasound. 2017; 20:1–9.12. Fichera A, Krane MK. Crohn’s disease: basic principles. Cham: Springer International Publishing;2015.13. Lee SS, Kim AY, Yang SK, et al. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009; 251:751–761.
Article14. Gatta G, Di Grezia G, Di Mizio V, et al. Crohn’s disease imaging: a review. Gastroenterol Res Pract. 2012; 2012:816920.
Article15. Maglinte DD, Chernish SM, Kelvin FM, O’Connor KW, Hage JP. Crohn disease of the small intestine: accuracy and relevance of enteroclysis. Radiology. 1992; 184:541–545.
Article16. Levine MS, Ramchandani P, Rubesin S. Practical fluoroscopy of the GI and GU tracts. Cambridge: Cambridge University Press;2012.17. Kelvin FM, Oddson TA, Rice RP, Garbutt JT, Bradenham BP. Double contrast barium enema in Crohn’s disease and ulcerative colitis. AJR Am J Roentgenol. 1978; 131:207–213.
Article18. Margulis AR. Editorial: the overlapping spectrum of radiologic signs in chronic ulcerative colitis and Crohn’s disease of the colon. Calif Med. 1973; 119:49–50.19. Carnevale Maffè G, Brunetti L, Formagnana P, Corazza GR. Ultrasonographic findings in Crohn’s disease. J Ultrasound. 2014; 18:37–49.
Article20. Hirche TO, Russler J, Schröder O, et al. The value of routinely performed ultrasonography in patients with Crohn disease. Scand J Gastroenterol. 2002; 37:1178–1183.
Article21. Calabrese E, Maaser C, Zorzi F, et al. Bowel ultrasonography in the management of Crohn’s disease: a review with recommendations of an international panel of experts. Inflamm Bowel Dis. 2016; 22:1168–1183.
Article22. Greenup AJ, Bressler B, Rosenfeld G. Medical imaging in small bowel Crohn’s disease-computer tomography enterography, magnetic resonance enterography, and ultrasound: “which one is the best for what?”. Inflamm Bowel Dis. 2016; 22:1246–1261.
Article23. Panés J, Bouzas R, Chaparro M, et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther. 2011; 34:125–145.
Article24. Rimola J. Cross-sectional imaging in Crohn’s disease. Cham: Springer;2019.25. Ripollés T, Martínez-Pérez MJ, Paredes JM, Vizuete J, GarcíaMartínez E, Jiménez-Restrepo DH. Contrast-enhanced ultrasound in the differentiation between phlegmon and abscess in Crohn’s disease and other abdominal conditions. Eur J Radiol. 2013; 82:e525–e531.
Article26. Conti CB, Giunta M, Gridavilla D, Conte D, Fraquelli M. Role of bowel ultrasound in the diagnosis and follow-up of patients with Crohn’s disease. Ultrasound Med Biol. 2017; 43:725–734.
Article27. Gasche C, Moser G, Turetschek K, Schober E, Moeschl P, Oberhuber G. Transabdominal bowel sonography for the detection of intestinal complications in Crohn’s disease. Gut. 1999; 44:112–117.
Article28. Pallotta N, Tomei E, Viscido A, et al. Small intestine contrast ultrasonography: an alternative to radiology in the assessment of small bowel disease. Inflamm Bowel Dis. 2005; 11:146–153.29. Calabrese E, La Seta F, Buccellato A, et al. Crohn’s disease: a comparative prospective study of transabdominal ultrasonography, small intestine contrast ultrasonography, and small bowel enema. Inflamm Bowel Dis. 2005; 11:139–145.
Article30. Parente F, Greco S, Molteni M, et al. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn’s disease: a prospective comparison with conventional ultrasound, X ray studies, and ileocolonoscopy. Gut. 2004; 53:1652–1657.
Article31. Calabrese E, Zorzi F, Pallone F. Ultrasound in Crohn’s disease. Curr Drug Targets. 2012; 13:1224–1233.
Article32. Pallotta N, Civitelli F, Di Nardo G, et al. Small intestine contrast ultrasonography in pediatric Crohn’s disease. J Pediatr. 2013; 163:778–784.
Article33. Farmer RG, Whelan G, Fazio VW. Long-term follow-up of patients with Crohn’s disease: relationship between the clinical pattern and prognosis. Gastroenterology. 1985; 88:1818–1825.34. Baumgart DC, Müller HP, Grittner U, et al. US-based real-time elastography for the detection of fibrotic gut tissue in patients with stricturing Crohn disease. Radiology. 2015; 275:889–899.
Article35. Dillman JR, Stidham RW, Higgins PD, et al. Ultrasound shear wave elastography helps discriminate low-grade from highgrade bowel wall fibrosis in ex vivo human intestinal specimens. J Ultrasound Med. 2014; 33:2115–2123.
Article36. Fraquelli M, Branchi F, Cribiù FM, et al. The role of ultrasound elasticity imaging in predicting ileal fibrosis in Crohn’s disease patients. Inflamm Bowel Dis. 2015; 21:2605–2612.
Article37. Rimola J, Capozzi N. Differentiation of fibrotic and inflammatory component of Crohn’s disease-associated strictures. Intest Res. 2020; 18:144–150.
Article38. Stewart LK, McGee J, Wilson SR. Transperineal and transvaginal sonography of perianal inflammatory disease. AJR Am J Roentgenol. 2001; 177:627–632.
Article39. Limberg B, Osswald B. Diagnosis and differential diagnosis of ulcerative colitis and Crohn’s disease by hydrocolonic sonography. Am J Gastroenterol. 1994; 89:1051–1057.40. Baumgart DC. Crohn’s disease and ulcerative colitis. Cham: Springer International Publishing AG;2017.41. Huprich JE, Fletcher JG. CT enterography: principles, technique and utility in Crohn’s disease. Eur J Radiol. 2009; 69:393–397.
Article42. Baker ME, Hara AK, Platt JF, Maglinte DD, Fletcher JG. CT enterography for Crohn’s disease: optimal technique and imaging issues. Abdom Imaging. 2015; 40:938–952.
Article43. Raman SP, Horton KM, Fishman EK. Computed tomography of Crohn’s disease: the role of three dimensional technique. World J Radiol. 2013; 5:193–201.
Article44. Deepak P, Fletcher JG, Fidler JL, Bruining DH. Computed tomography and magnetic resonance enterography in Crohn’s disease: assessment of radiologic criteria and endpoints for clinical practice and trials. Inflamm Bowel Dis. 2016; 22:2280–2288.
Article45. Spektor M, Mathur M, Santacana G, et al. Does MR enterography offer added value after a recent CT in the evaluation of abdominal pain in Crohn’s disease patients? Clin Imaging. 2019; 54:78–83.
Article46. Chang CW, Wong JM, Tung CC, Shih IL, Wang HY, Wei SC. Intestinal stricture in Crohn’s disease. Intest Res. 2015; 13:19–26.
Article47. Cicero G, Ascenti G, Albrecht MH, et al. Extra-abdominal dual-energy CT applications: a comprehensive overview. Radiol Med. 2020; 125:384–397.
Article48. Kim YS, Kim SH, Ryu HS, Han JK. Iodine quantification on spectral detector-based dual-energy CT enterography: correlation with Crohn’s disease activity index and external validation. Korean J Radiol. 2018; 19:1077–1088.
Article49. Dane B, Duenas S, Han J, et al. Crohn’s disease activity quantified by iodine density obtained from dual-energy computed tomography enterography. J Comput Assist Tomogr. 2020; 44:242–247.
Article50. Park MJ, Lim JS. Computed tomography enterography for evaluation of inflammatory bowel disease. Clin Endosc. 2013; 46:327–366.
Article51. Bruining DH, Zimmermann EM, Loftus EV Jr, et al. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance enterography in patients with small bowel Crohn’s disease. Radiology. 2018; 286:776–799.
Article52. Elsayes KM, Al-Hawary MM, Jagdish J, Ganesh HS, Platt JF. CT enterography: principles, trends, and interpretation of findings. Radiographics. 2010; 30:1955–1970.
Article53. Cicero G, Mondello S, Wichmann JL, et al. Fast magnetic resonance enterography protocol for the evaluation of patients with Crohn’s disease: a pilot study. J Clin Imaging Sci. 2020; 10:25.
Article54. Lee JH, Park YE, Seo N, et al. Magnetic resonance enterography predicts the prognosis of Crohn’s disease. Intest Res. 2018; 16:445–457.
Article55. Kim SH. Computed tomography enterography and magnetic resonance enterography in the diagnosis of Crohn’s disease. Intest Res. 2015; 13:27–38.
Article56. Ohtsuka K, Takenaka K, Kitazume Y, et al. Magnetic resonance enterography for the evaluation of the deep small intestine in Crohn’s disease. Intest Res. 2016; 14:120–126.
Article57. Prassopoulos P, Papanikolaou N, Grammatikakis J, Rousomoustakaki M, Maris T, Gourtsoyiannis N. MR enteroclysis imaging of Crohn disease. Radiographics. 2001; 21 Spec No:S161–S172.
Article58. Moy MP, Sauk J, Gee MS. The role of MR Enterography in assessing Crohn’s disease activity and treatment response. Gastroenterol Res Pract. 2016; 2016:8168695.
Article59. Mazziotti S, Blandino A. MR enterography. Berlin: Springer;2014.60. Taylor SA, Mallett S, Bhatnagar G, et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease (METRIC): a multicentre trial. Lancet Gastroenterol Hepatol. 2018; 3:548–558.61. Pita I, Magro F. Advanced imaging techniques for small bowel Crohn’s disease: what does the future hold? Therap Adv Gastroenterol. 2018; 11:1756283–X18757185.
Article62. D’Angelo T, Gallizzi R, Romano C, Cicero G, Mazziotti S. Magnetic resonance enterography findings of intestinal Behçet disease in a child. Case Rep Radiol. 2017; 2017:8061648.
Article63. Cicero G, Blandino A, D’Angelo T, et al. Magnetic resonance enterography appraisal of lupus enteritis: a case report. Radiol Case Rep. 2018; 13:915–919.
Article64. Cicero G, Ascenti G, Bottari A, Catanzariti F, Blandino A, Mazziotti S. MR enterography: what is next after Crohn’s disease? Jpn J Radiol. 2019; 37:511–517.
Article65. Erden A. MRI of anal canal: normal anatomy, imaging protocol, and perianal fistulas: part 1. Abdom Radiol (NY). 2018; 43:1334–1352.
Article66. Thipphavong S, Costa AF, Ali HA, Wang DC, Brar MS, Jhaveri KS. Structured reporting of MRI for perianal fistula. Abdom Radiol (NY). 2019; 44:1295–1305.
Article67. Guglielmo FF, Anupindi SA, Fletcher JG, et al. Small bowel Crohn disease at CT and MR enterography: imaging atlas and glossary of terms. Radiographics. 2020; 40:354–375.
Article68. Gücer FI, Senturk S, Özkanli S, Yilmabasar MG, Köroglu GA, Acar M. Evaluation of Crohn’s disease activity by MR enterography: derivation and histopathological comparison of an MR-based activity index. Eur J Radiol. 2015; 84:1829–1834.
Article69. Ordás I, Rimola J, Alfaro I, et al. Development and validation of a simplified magnetic resonance index of activity for Crohn’s disease. Gastroenterology. 2019; 157:432–439.
Article70. Steward MJ, Punwani S, Proctor I, et al. Non-perforating small bowel Crohn’s disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol. 2012; 81:2080–2088.
Article71. Makanyanga JC, Pendsé D, Dikaios N, et al. Evaluation of Crohn’s disease activity: initial validation of a magnetic resonance enterography global score (MEGS) against faecal calprotectin. Eur Radiol. 2014; 24:277–287.
Article72. Kaushal P, Somwaru AS, Charabaty A, Levy AD. MR enterography of inflammatory bowel disease with endoscopic correlation. Radiographics. 2017; 37:116–131.
Article73. Heller MT, Haarer KA, Itri JN, Sun X. Duodenum: MDCT of acute conditions. Clin Radiol. 2014; 69:e48–e55.
Article74. Lightner AL. Duodenal Crohn’s disease. Inflamm Bowel Dis. 2018; 24:546–551.
Article75. Song DJ, Whang IS, Choi HW, Jeong CY, Jung SH. Crohn’s disease confined to the duodenum: a case report. World J Clin Cases. 2016; 4:146–150.
Article76. Pimentel AM, Rocha R, Santana GO. Crohn’s disease of esophagus, stomach and duodenum. World J Gastrointest Pharmacol Ther. 2019; 10:35–49.
Article77. Cicero G, D’Angelo T, Bottari A, et al. Superior mesenteric artery syndrome in patients with Crohn’s disease: a description of 2 cases studied with a novel magnetic resonance enterography (MRE) procedure. Am J Case Rep. 2018; 19:431–437.
Article78. Kim YS, Jung SA. Crohn’s disease with jejunal involvement as a predictor of long-term clinical outcomes. Gut Liver. 2018; 12:3–4.
Article79. Caprilli R. Why does Crohn’s disease usually occur in terminal ileum? J Crohns Colitis. 2008; 2:352–356.
Article80. Goulart RA, Barbalho SM, Gasparini RG, de Carvalho AC. Facing terminal ileitis: going beyond Crohn’s disease. Gastroenterology Res. 2016; 9:1–9.81. Bojic D, Markovic S. Terminal ileitis is not always Crohn’s disease. Ann Gastroenterol. 2011; 24:271–275.82. Nehra AK, Sheedy SP, Wells ML, et al. Imaging findings of ileal inflammation at computed tomography and magnetic resonance enterography: what do they mean when ileoscopy and biopsy are negative? J Crohns Colitis. 2020; 14:455–464.
Article83. Hedrick TL, Friel CM. Colonic Crohn disease. Clin Colon Rectal Surg. 2013; 26:84–89.84. Subramanian S, Ekbom A, Rhodes JM. Recent advances in clinical practice: a systematic review of isolated colonic Crohn’s disease: the third IBD? Gut. 2017; 66:362–381.
Article85. Soucy G, Wang HH, Farraye FA, et al. Clinical and pathological analysis of colonic Crohn’s disease, including a subgroup with ulcerative colitis-like features. Mod Pathol. 2012; 25:295–307.
Article86. Hristova L, Soyer P, Hoeffel C, et al. Colorectal cancer in inflammatory bowel diseases: CT features with pathological correlation. Abdom Imaging. 2013; 38:421–435.
Article87. Kim KJ, Lee Y, Park SH, et al. Diffusion-weighted MR enterography for evaluating Crohn’s disease: how does it add diagnostically to conventional MR enterography? Inflamm Bowel Dis. 2015; 21:101–109.88. Kelley KA, Kaur T, Tsikitis VL. Perianal Crohn’s disease: challenges and solutions. Clin Exp Gastroenterol. 2017; 10:39–46.89. Wedemeyer J, Kirchhoff T, Sellge G, et al. Transcutaneous perianal sonography: a sensitive method for the detection of perianal inflammatory lesions in Crohn’s disease. World J Gastroenterol. 2004; 10:2859–2863.
Article90. Sun Y, Cui LG, Liu JB, Wang JR, Ping H, Chen ZW. Utility of 360° real-time endoanal sonography for evaluation of perianal fistulas. J Ultrasound Med. 2018; 37:93–98.
Article91. Arora U, Kedia S, Garg P, et al. Colonic Crohn’s disease is associated with less aggressive disease course than ileal or ileocolonic disease. Dig Dis Sci. 2018; 63:1592–1599.
Article92. Hwang JY, Yoon HK, Kim WK, et al. Transperineal ultrasonography for evaluation of the perianal fistula and abscess in pediatric Crohn disease: preliminary study. Ultrasonography. 2014; 33:184–190.93. Liang C, Lu Y, Zhao B, Du Y, Wang C, Jiang W. Imaging of anal fistulas: comparison of computed tomographic fistulography and magnetic resonance imaging. Korean J Radiol. 2014; 15:712–723.
Article94. Liang C, Jiang W, Zhao B, Zhang Y, Du Y, Lu Y. CT imaging with fistulography for perianal fistula: does it really help the surgeon? Clin Imaging. 2013; 37:1069–1076.95. O’Malley RB, Al-Hawary MM, Kaza RK, Wasnik AP, Liu PS, Hussain HK. Rectal imaging: part 2, perianal fistula evaluation on pelvic MRI--what the radiologist needs to know. AJR Am J Roentgenol. 2012; 199:W43–W53.96. Balcı S, Onur MR, Karaosmanoğlu AD, et al. MRI evaluation of anal and perianal diseases. Diagn Interv Radiol. 2019; 25:21–27.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Crohn's Disease Showing a Skin Lesion with a Cobblestone-like Appearance in the Perianal Region
- Computed Tomography Enterography and Magnetic Resonance Enterography in the Diagnosis of Crohn's Disease
- A Case of Crohns Disease Misdiagnosed as Intestinal Tuberculosis
- Isolated Crohn's Disease of Stomach A case report and review of the literature
- Radiologic images of complications of Crohn’s disease