Cancer Res Treat.
2021 Oct;53(4):1184-1194. 10.4143/crt.2020.289.
Effectiveness and Safety of Clofarabine Monotherapy or Combination Treatment in Relapsed/Refractory Childhood Acute Lymphoblastic Leukemia: A Pragmatic, Non-interventional Study in Korea
- Affiliations
-
- 1Department of Pediatrics, Seoul National University Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Pediatrics, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Division of Pediatric Hematology/Oncology, Department of Pediatrics, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea
- 4Division of Pediatric Hematology and Oncology, Department of Pediatrics, Yonsei University Health System, Yonsei University College of Medicine, Seoul, Korea
- 5Department of Pediatrics, Pusan National University Children’s Hospital, Pusan National University School of Medicine, Yangsan, Korea
- 6Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 7Department of Pediatrics, Chonnam National University Hwasun Hospital, Chonnam National University Medical School, Hwasun, Korea
- 8Department of Pediatrics, Gachon University, Gil Medical Center, Incheon, Korea
- 9Sanofi Aventis, Seoul, Korea
- KMID: 2521593
- DOI: http://doi.org/10.4143/crt.2020.289
Abstract
- Purpose
Effectiveness and safety of clofarabine (one of the treatment mainstays in pediatric patients with relapsed/refractory acute lymphoblastic leukemia [ALL]) was assessed in Korean pediatric patients with ALL to facilitate conditional coverage with evidence development.
Materials and Methods
In this multicenter, prospective, observational study, patients receiving clofarabine as mono/combination therapy were followed up every 4-6 weeks for 6 months or until hematopoietic stem cell transplantation (HSCT). Response rates, survival outcomes, and adverse events were assessed.
Results
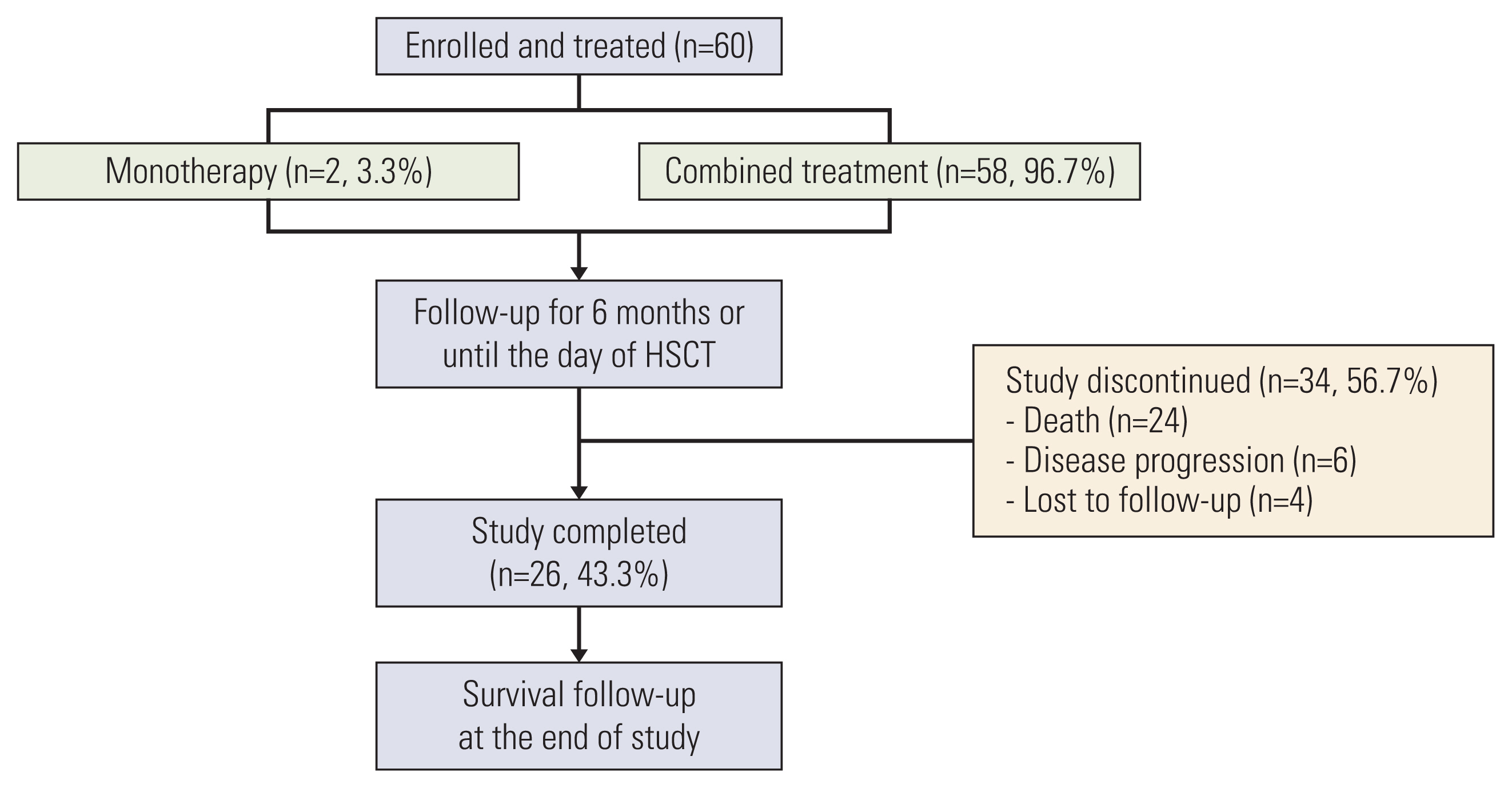
Sixty patients (2-26 years old; 65% B-cell ALL, received prior ≥ 2 regimen, 68.3% refractory to previous regimen) were enrolled and treated with at least one dose of clofarabine; of whom 26 (43.3%) completed 6 months of follow-up after the last dose of clofarabine. Fifty-eight patients (96.7%) received clofarabine combination therapy. Overall remission rate (complete remission [CR] or CR without platelet recovery [CRp]) was 45.0% (27/60; 95% confidence interval [CI], 32.4 to 57.6) and the overall response rate (CR, CRp, or partial remission [PR]) was 46.7% (28/60; 95% CI, 34.0 to 59.3), with 11 (18.3%), 16 (26.7%), and one (1.7%) patients achieving CR, CRp, and PR, respectively. The median time to remission was 5.1 weeks (95% CI, 4.7 to 6.1). Median duration of remission was 16.6 weeks (range, 2.0 to 167.6 weeks). Sixteen patients (26.7%) proceeded to HSCT. There were 24 deaths; 14 due to treatment-emergent adverse events.
Conclusion
Remission with clofarabine was observed in approximately half of the study patients who had overall expected safety profile; however, there was no favorable long-term survival outcome in this study.
Figure
Reference
-
References
1. Kato M, Manabe A. Treatment and biology of pediatric acute lymphoblastic leukemia. Pediatr Int. 2018; 60:4–12.
Article2. Boer JM, den Boer ML. BCR-ABL1-like acute lymphoblastic leukaemia: from bench to bedside. Eur J Cancer. 2017; 82:203–18.3. Stary J, Hrusak O. Recent advances in the management of pediatric acute lymphoblastic leukemia [version 1; peer review: 2 approved]. F1000Res. 2016; 5:2635.4. Park HJ, Moon EK, Yoon JY, Oh CM, Jung KW, Park BK, et al. Incidence and survival of childhood cancer in Korea. Cancer Res Treat. 2016; 48:869–82.
Article5. Kim H. Recent advances in the treatment of pediatric acute leukemia. J Korean Med Assoc. 2016; 59:690–7.
Article6. Pui CH. Recent research advances in childhood acute lymphoblastic leukemia. J Formos Med Assoc. 2010; 109:777–87.
Article7. Ju HY, Hong CR, Shin HY. Advancements in the treatment of pediatric acute leukemia and brain tumor: continuous efforts for 100% cure. Korean J Pediatr. 2014; 57:434–9.8. Ceppi F, Duval M, Leclerc JM, Laverdiere C, Delva YL, Cellot S, et al. Improvement of the outcome of relapsed or refractory acute lymphoblastic leukemia in children using a risk-based treatment strategy. PLoS One. 2016; 11:e0160310.
Article9. Fuster JL. Current approach to relapsed acute lymphoblastic leukemia in children. World J Hematol. 2014; 3:49–70.
Article10. Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010; 28:3730–8.
Article11. Inagaki J, Fukano R, Noguchi M, Kurauchi K, Tanioka S, Okamura J. Hematopoietic stem cell transplantation following unsuccessful salvage treatment for relapsed acute lymphoblastic leukemia in children. Pediatr Blood Cancer. 2015; 62:674–9.
Article12. Jeha S, Kantarjian H. Clofarabine for the treatment of acute lymphoblastic leukemia. Expert Rev Anticancer Ther. 2007; 7:113–8.
Article13. Raetz EA, Bhatla T. Where do we stand in the treatment of relapsed acute lymphoblastic leukemia? Hematology Am Soc Hematol Educ Program. 2012; 2012:129–36.
Article14. Pession A, Masetti R, Kleinschmidt K, Martoni A. Use of clofarabine for acute childhood leukemia. Biologics. 2010; 4:111–8.15. Yoo KH, Chung NG, Cho B, Kang HJ, Shin HY, Im HJ, et al. A multicenter retrospective analysis on the treatment pattern and outcome in relapsed/refractory childhood acute lymphoblastic leukemia. Clin Pediatr Hematol Oncol. 2017; 24:101–6.
Article16. Yoo SL, Kim DJ, Lee SM, Kang WG, Kim SY, Lee JH, et al. Improving patient access to new drugs in South Korea: evaluation of the national drug formulary system. Int J Environ Res Public Health. 2019; 16:e288.
Article17. Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) Co-operative Group. Br J Haematol. 1976; 33:451–8.
Article18. Jeha S, Gaynon PS, Razzouk BI, Franklin J, Kadota R, Shen V, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol. 2006; 24:1917–23.
Article19. Hijiya N, Thomson B, Isakoff MS, Silverman LB, Steinherz PG, Borowitz MJ, et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood. 2011; 118:6043–9.
Article20. Locatelli F, Testi AM, Bernardo ME, Rizzari C, Bertaina A, Merli P, et al. Clofarabine, cyclophosphamide and etoposide as single-course re-induction therapy for children with refractory/multiple relapsed acute lymphoblastic leukaemia. Br J Haematol. 2009; 147:371–8.
Article21. Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008; 22:2142–50.
Article22. Bonate PL, Cunningham CC, Gaynon P, Jeha S, Kadota R, Lam GN, et al. Population pharmacokinetics of clofarabine and its metabolite 6-ketoclofarabine in adult and pediatric patients with cancer. Cancer Chemother Pharmacol. 2011; 67:875–90.
Article23. Barreto JN, McCullough KB, Ice LL, Smith JA. Antineoplastic agents and the associated myelosuppressive effects: a review. J Pharm Pract. 2014; 27:440–6.24. Nordvig J, Aagaard T, Daugaard G, Brown P, Sengelov H, Lundgren J, et al. Febrile neutropenia and long-term risk of infection among patients treated with chemotherapy for malignant diseases. Open Forum Infect Dis. 2018; 5:ofy255.
Article25. Food and Drug Administration. Highlights of prescribing information: Clolar (clofarabine) [Internet]. Silver Spring MD: Food and Drug Administration;2015. [cited 2021 Jan 2]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021673s024lbl.pdf .26. von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016; 34:4381–9.
Article27. Jabbour E, Dull J, Yilmaz M, Khoury JD, Ravandi F, Jain N, et al. Outcome of patients with relapsed/refractory acute lymphoblastic leukemia after blinatumomab failure: no change in the level of CD19 expression. Am J Hematol. 2018; 93:371–4.
Article28. Hucks G, Rheingold SR. The journey to CAR T cell therapy: the pediatric and young adult experience with relapsed or refractory B-ALL. Blood Cancer J. 2019; 9:10.
Article29. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018; 378:439–48.
Article30. Kenderian SS, Porter DL, Gill S. Chimeric antigen receptor T cells and hematopoietic cell transplantation: how not to put the CART before the horse. Biol Blood Marrow Transplant. 2017; 23:235–46.
Article31. Forsberg MH, Das A, Saha K, Capitini CM. The potential of CAR T therapy for relapsed or refractory pediatric and young adult B-cell ALL. Ther Clin Risk Manag. 2018; 14:1573–84.
Article32. Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019; 16:372–85.
Article33. Campana D, Pui CH. Minimal residual disease-guided therapy in childhood acute lymphoblastic leukemia. Blood. 2017; 129:1913–8.
Article34. Lovisa F, Zecca M, Rossi B, Campeggio M, Magrin E, Giarin E, et al. Pre- and post-transplant minimal residual disease predicts relapse occurrence in children with acute lymphoblastic leukaemia. Br J Haematol. 2018; 180:680–93.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Ifosfamide and Etoposide in Relapsed Refractory Childhood Acute Lymphoblastic Leukemia
- Advances in the Treatment of Childhood Acute Lymphoblastic Leukemia
- Meeting Report: 2009 Symposium on Childhood Acute Lymphoblastic Leukemia - Update on the Diagnosis and Treatment for Acute Lymphoblastic Leukemia in Childhood & Adolescence; Seoul; Korea; June 27, 2009
- A Multicenter Retrospective Analysis on the Treatment Pattern and Outcome in Relapsed/Refractory Childhood Acute Lymphoblastic Leukemia
- Recent Advance in Treatment of Acute Lymphoblastic Leukemia in Childhood