Korean J Physiol Pharmacol.
2021 Nov;25(6):497-506. 10.4196/kjpp.2021.25.6.497.
Depilatory creams increase the number of hair follicles, and dermal fibroblasts expressing interleukin-6, tumor necrosis factor-α, and tumor necrosis factor-β in mouse skin
- Affiliations
-
- 1Institute of Medicine, Chung Shan Medical University, Taichung 40221, Taiwan
- 2Department of Biochemistry, School of Medicine, College of Medicine, Chung Shan Medical University, Taichung 40221, Taiwan
- 3Department of Clinical Biochemistry, Chung Shan Medical University Hospital, Taichung 40221, Taiwan
- 4Department of Cosmetic Science, Chang Gung University of Science and Technology, Taoyuan 33382, Taiwan
- KMID: 2521475
- DOI: http://doi.org/10.4196/kjpp.2021.25.6.497
Abstract
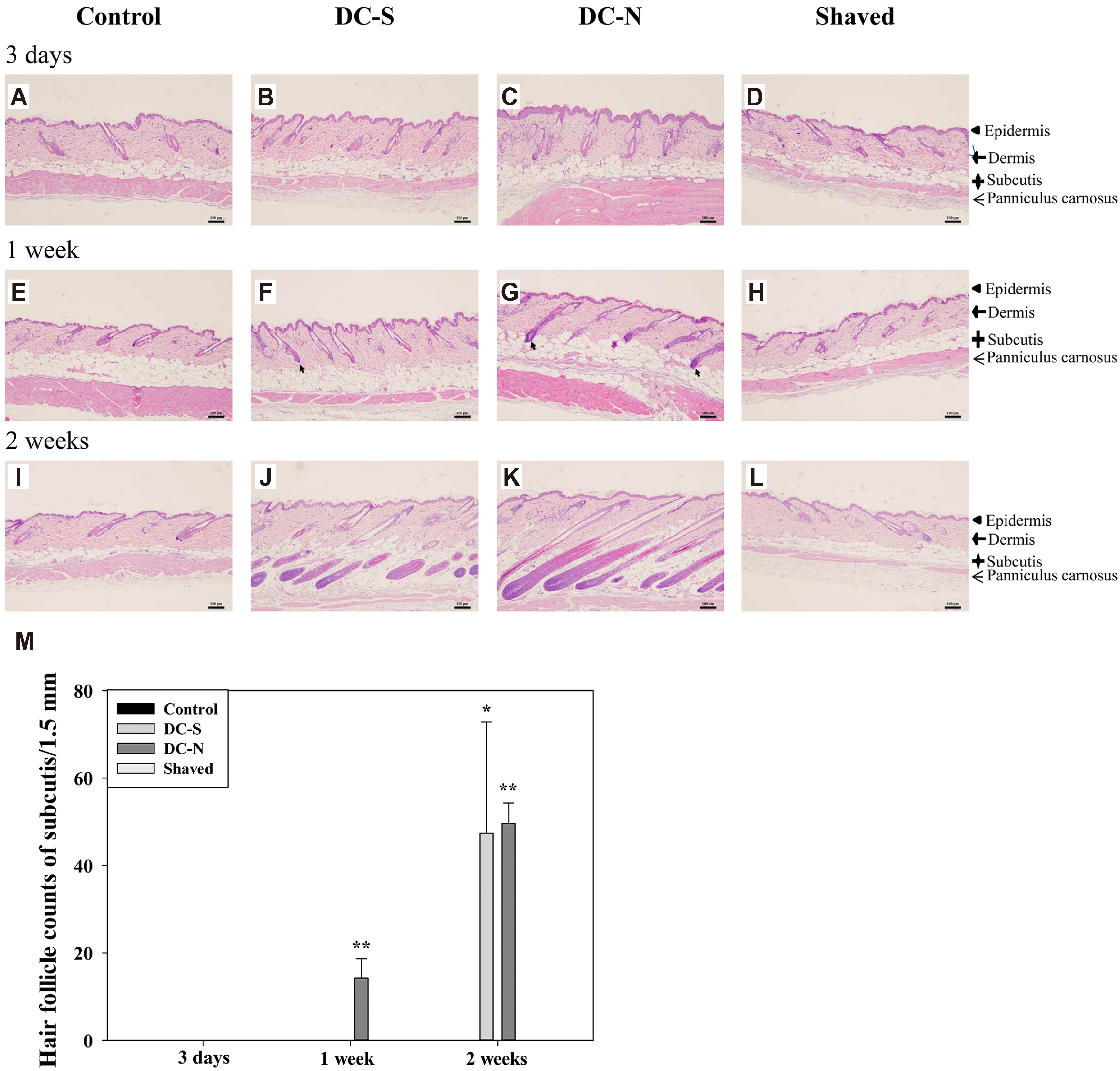
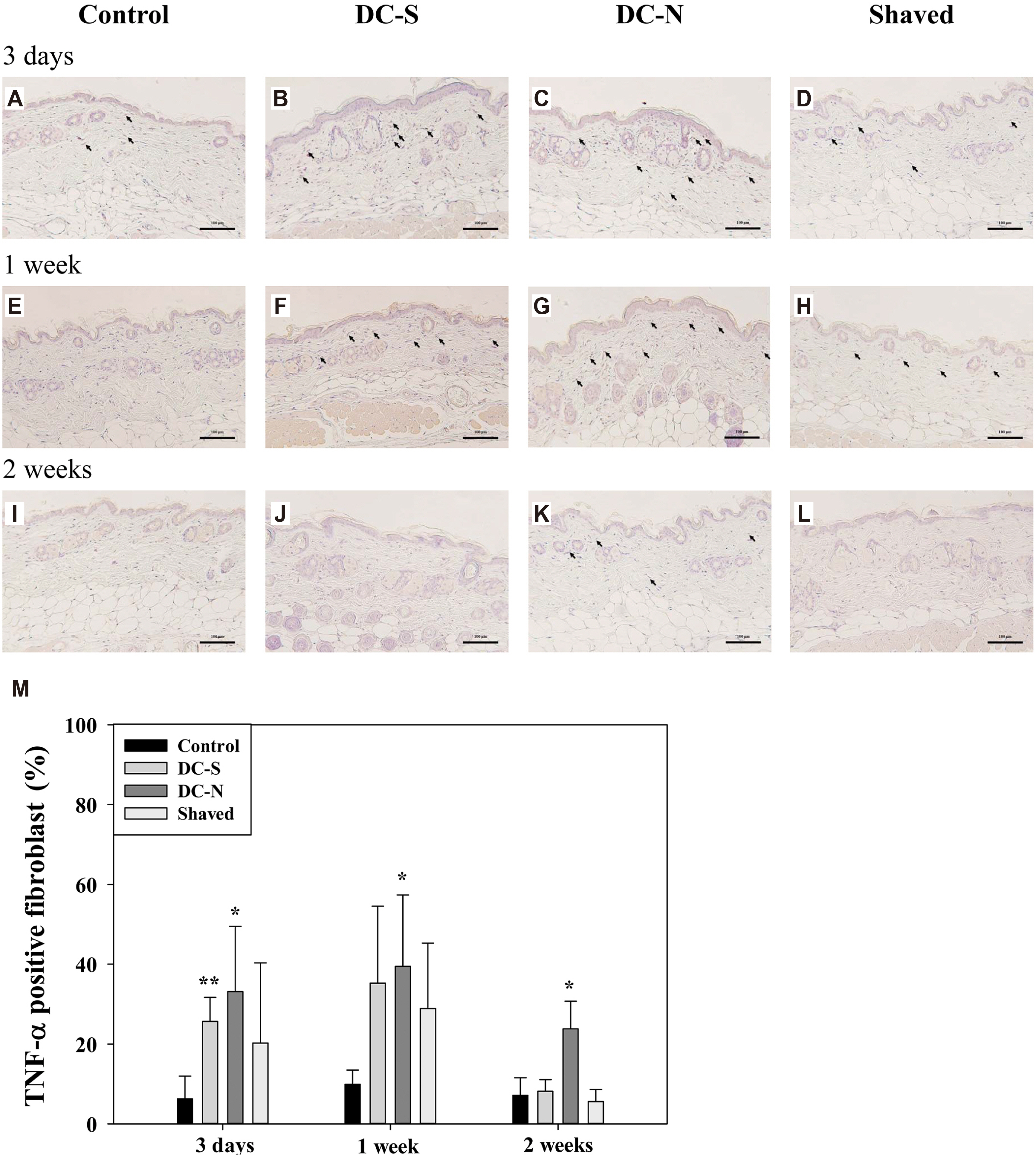
- Besides using for hair removal, depilatory agents have been considered to be used as a penetration enhancer for transepidermal drug delivery. To examine the effect in hair follicles (HFs), two commercially available depilatory creams were tested on the dorsal skin of mice to monitor the effect deep into the skin structure. Fifteen male BALB/c mice were used in this study. Depilatory creams were applied to the dorsal skin of the same animal using shaved and untouched treatments as controls to minimize individual differences. Skin samples were collected at three days, one week and two weeks (n = 5 for each) after the treatment, and subjected for hematoxylin-eosin staining, and immunohistochemical analysis for proinflammatory cytokines. The morphological examination showed an increase in the thickness of epidermal layer of the depilatory cream-treated skin at early time points and in the subcutis at two weeks. Depilatory cream promoted entry of anagen phase and increased the number of hair follicles in the subcutis at one and two weeks. Immunohistochemistry showed elevated percentages of dermal fibroblasts expressing interleukin-6, tumor necrosis factor-α, and tumor necrosis factor-β. Shaving process increased the thickness of epidermis and dermis as depilatory creams did, but did neither induce the expression of proinflammatory cytokines in the dermal fibroblasts nor the number of HFs. The results suggested that the commercially available depilatory creams caused a transient minor inflammatory response of the skin and increased the levels of cytokines that might subsequently affect hair growth.
Figure
Reference
-
1. Clausen T, Schwan-Jonczyk A, Lang G, Schuh W, Liebscher KD, Springob C, Franzke M, Balzer W, Imhoff S, Maresch G, Bimczok R. Bellussi G, Bohnet M, Bus J, Drauz K, Greim H, Jackel KP, Karst U, Kleemann A, Kreysa G, Laird T, Meier W, Ottow E, Roper M, Scholtz J, Sundmacher K, Ulber R, Wietelmann U, editors. 2006. Hair preparations. Ullmann's encyclopedia of industrial chemistry. 7th ed. Wiley-VCH Verlag GmbH & Co. KGaA.;Weinheim: p. 204–241. https://onlinelibrary.wiley.com/doi/10.1002/14356007.a12_571.pub2. DOI: 10.1002/14356007.a12_571.pub2.
Article2. Pohl S, Varco J, Wallace P, Wolfram LJ. Othmer K, editor. 2013. Hair preparations. Kirk-Othmer chemical technology of cosmetics. 5th ed. John Wiley & Sons;Hoboken (NJ): p. 85–122. https://www.wiley.com/en-us/Kirk+Othmer+Chemical+Technology+of+Cosmetics-p-9781118406922.
Article3. Kushida K, Masaki K, Matsumura M, Ohshima T, Yoshikawa H, Takada K, Muranishi S. 1984; Application of calcium thioglycolate to improve transdermal delivery of theophylline in rats. Chem Pharm Bull (Tokyo). 32:268–274. DOI: 10.1248/cpb.32.268. PMID: 6722953.
Article4. Zakzewski CA, Wasilewski J, Cawley P, Ford W. 1998; Transdermal delivery of regular insulin to chronic diabetic rats: effect of skin preparation and electrical enhancement. J Control Release. 50:267–272. DOI: 10.1016/S0168-3659(97)00143-0. PMID: 9685893.
Article5. Kanikkannan N, Singh J, Ramarao P. 1999; Transdermal iontophoretic delivery of bovine insulin and monomeric human insulin analogue. J Control Release. 59:99–105. DOI: 10.1016/S0168-3659(98)00184-9. PMID: 10210726.
Article6. Lee JN, Jee SH, Chan CC, Lo W, Dong CY, Lin SJ. 2008; The effects of depilatory agents as penetration enhancers on human stratum corneum structures. . J Invest Dermatol. 128:2240–2247. DOI: 10.1038/jid.2008.82. PMID: 18401425.
Article7. Duit R, Hawkins TJ, Määttä A. 2019; Depilatory chemical thioglycolate affects hair cuticle and cortex, degrades epidermal cornified envelopes and induces proliferation and differentiation responses in keratinocytes. Exp Dermatol. 28:76–79. DOI: 10.1111/exd.13838. PMID: 30417461.
Article8. Paus R, Maurer M, Slominski A, Czarnetzki BM. 1994; Mast cell involvement in murine hair growth. Dev Biol. 163:230–240. DOI: 10.1006/dbio.1994.1139. PMID: 8174779.
Article9. Chase HB, Eaton GJ. 1959; The growth of hair follicles in waves. Ann N Y Acad Sci. 83:365–368. DOI: 10.1111/j.1749-6632.1960.tb40912.x. PMID: 13809378.
Article10. Datta K, Singh AT, Mukherjee A, Bhat B, Ramesh B, Burman AC. 2009; Eclipta alba extract with potential for hair growth promoting activity. J Ethnopharmacol. 124:450–456. DOI: 10.1016/j.jep.2009.05.023. PMID: 19481595.
Article11. Chase HB. 1954; Growth of the hair. Physiol Rev. 34:113–126. DOI: 10.1152/physrev.1954.34.1.113. PMID: 13120379.
Article12. Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL, Sugawara T, Luster MI. 2000; Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 14:2525–2531. DOI: 10.1096/fj.00-0073com. PMID: 11099471.
Article13. Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. 2003; Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 73:713–721. DOI: 10.1189/jlb.0802397. PMID: 12773503.
Article14. Pu CM, Chen YC, Chen YC, Lee TL, Peng YS, Chen SH, Yen YH, Chien CL, Hsieh JH, Chen YL. 2019; Interleukin-6 from adipose-derived stem cells promotes tissue repair by the increase of cell proliferation and hair follicles in ischemia/reperfusion-treated skin flaps. Mediators Inflamm. 2019:2343867. DOI: 10.1155/2019/2343867. PMID: 31814799. PMCID: PMC6877947.
Article15. Wu YF, Wang SH, Wu PS, Fan SM, Chiu HY, Tsai TH, Lin SJ. 2015; Enhancing hair follicle regeneration by nonablative fractional laser: assessment of irradiation parameters and tissue response. Lasers Surg Med. 47:331–341. DOI: 10.1002/lsm.22330. PMID: 25866259.
Article16. Philpott MP, Sanders DA, Bowen J, Kealey T. 1996; Effects of interleukins, colony-stimulating factor and tumour necrosis factor on human hair follicle growth in vitro: a possible role for interleukin-1 and tumour necrosis factor-alpha in alopecia areata. Br J Dermatol. 135:942–948. DOI: 10.1046/j.1365-2133.1996.d01-1099.x. PMID: 8977716.17. Sayama K, Kajiya K, Sugawara K, Sato S, Hirakawa S, Shirakata Y, Hanakawa Y, Dai X, Ishimatsu-Tsuji Y, Metzger D, Chambon P, Akira S, Paus R, Kishimoto J, Hashimoto K. 2010; Inflammatory mediator TAK1 regulates hair follicle morphogenesis and anagen induction shown by using keratinocyte-specific TAK1-deficient mice. PLoS One. 5:e11275. DOI: 10.1371/journal.pone.0011275. PMID: 20585657. PMCID: PMC2890581.
Article18. Heo SC, Jeon ES, Lee IH, Kim HS, Kim MB, Kim JH. 2011; Tumor necrosis factor-α-activated human adipose tissue-derived mesenchymal stem cells accelerate cutaneous wound healing through paracrine mechanisms. J Invest Dermatol. 131:1559–1567. DOI: 10.1038/jid.2011.64. PMID: 21451545.
Article19. Seiberg M, Marthinuss J, Stenn KS. 1995; Changes in expression of apoptosis-associated genes in skin mark early catagen. J Invest Dermatol. 104:78–82. DOI: 10.1111/1523-1747.ep12613555. PMID: 7798646.
Article20. Osaka N, Takahashi T, Murakami S, Matsuzawa A, Noguchi T, Fujiwara T, Aburatani H, Moriyama K, Takeda K, Ichijo H. 2007; ASK1-dependent recruitment and activation of macrophages induce hair growth in skin wounds. J Cell Biol. 176:903–909. DOI: 10.1083/jcb.200611015. PMID: 17389227. PMCID: PMC2064076.
Article21. Kluger N, Garat H. 2010; Transient localized hypertrichosis on a temporary henna tattoo. Contact Dermatitis. 62:188–189. DOI: 10.1111/j.1600-0536.2009.01691.x. PMID: 20565510.
Article22. Seo JA, Bae IH, Jang WH, Kim JH, Bak SY, Han SH, Park YH, Lim KM. 2012; Hydrogen peroxide and monoethanolamine are the key causative ingredients for hair dye-induced dermatitis and hair loss. J Dermatol Sci. 66:12–19. DOI: 10.1016/j.jdermsci.2011.12.015. PMID: 22269445.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Fibroblast-derived Cytokines in Melasma
- Regulation of mouse macrophage Fc receptor-mediated phagocytosis by interferon-r, lipid a and tumor necrosis factor
- Changes of Serum Tumor Necrosis Factor a and Interleukin 1B in the Sepsis of Neonates
- Immunohistochemical studies of Interleukin-8 and Tumor necrosis factor-alpha on psoriatic lesions
- Secretion of interleukin-1, tumor necrosis factor-alpha and expression of HLA-DR antigen by human cord blood monocytes