Anat Cell Biol.
2021 Sep;54(3):350-360. 10.5115/acb.20.299.
Ibrutinib reduces neutrophil infiltration, preserves neural tissue and enhances locomotor recovery in mouse contusion model of spinal cord injury
- Affiliations
-
- 1Department of Anatomical Sciences, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
- 2Department of Neuroscience, School of Advanced Medical Sciences and Technologies, Shiraz University of Medical Sciences, Shiraz, Iran
- 3Department of Neurosurgery, McKnight Brain Institute, University of Florida, Gainesville, FL, USA
- KMID: 2521048
- DOI: http://doi.org/10.5115/acb.20.299
Abstract
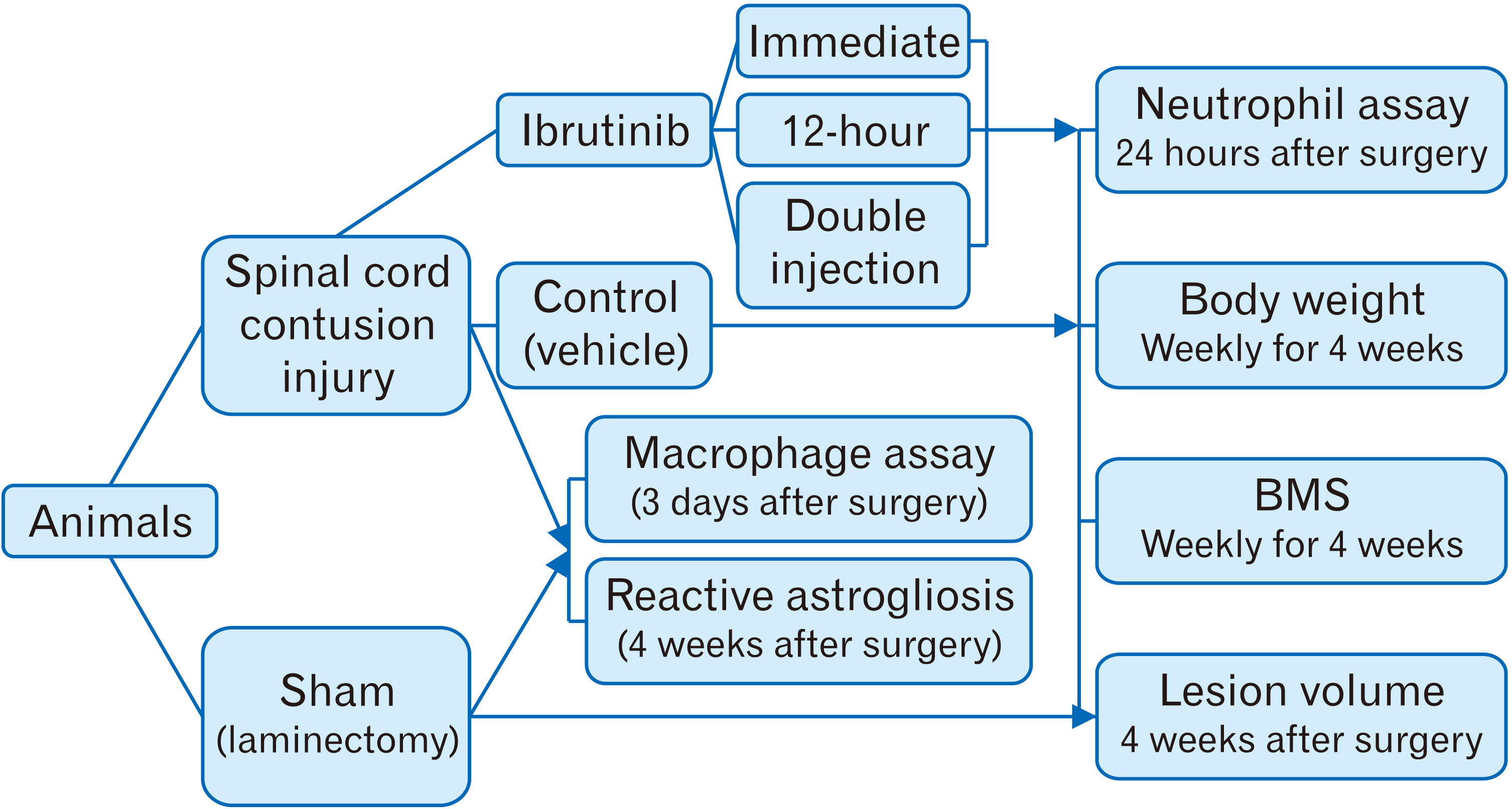
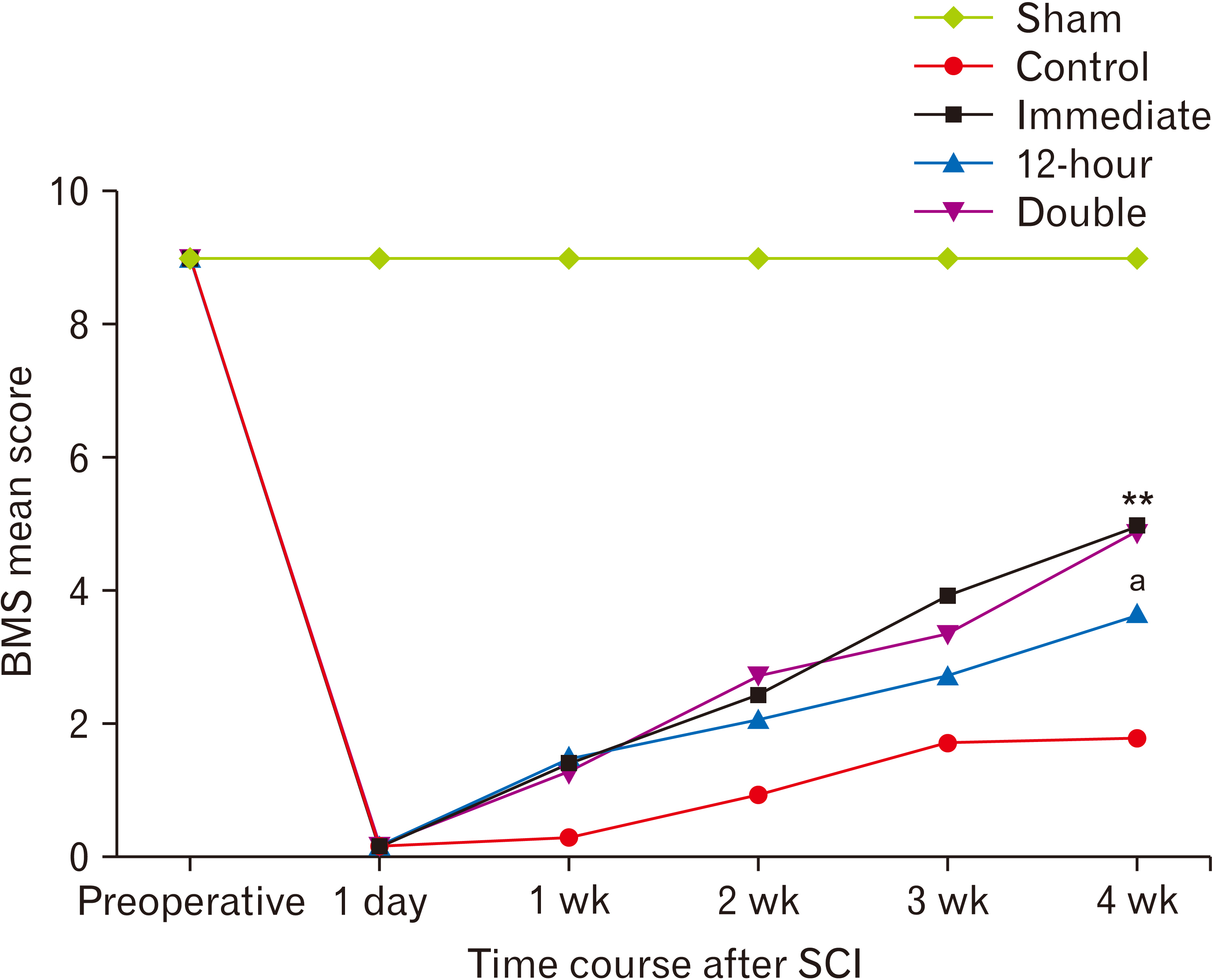
- Following acute spinal cord injury (SCI), excessive recruitment of neutrophils can result in inflammation, neural tissue loss and exacerbation of neurological outcomes. Ibrutinib is a bruton’s tyrosine kinase inhibitor in innate immune cells such as the neutrophils that diminishes their activation and influx to the site of injury. The present study evaluated the efficacy of ibrutinib administration in the acute phase of SCI on neural tissue preservation and locomotor recovery. Ibrutinib was delivered intravenously at 3.125 mg/kg either immediately, 12 hours after, or both immediately and 12 hours after SCI induction in adult male C57BL/6 mice. Neutrophil influx into the lesion area was evaluated 24 hours following SCI using light microscopy and immunohistochemistry methods. Animals’ body weight changes were recorded, and their functional motor recovery was assessed based on the Basso mouse scale during 28 days after treatment. Finally, spinal cord lesion volume was estimated by an unbiased stereological method. While animals’ weight in the control group started to increase one week after injury, it stayed unchanged in treatment groups. However, the double injection of ibrutinib led to a significantly lower body weight compared to the control group at 4 weeks post-injury. Mean neutrophil counts per visual field and the lesion volume were significantly decreased in all ibrutinib-treated groups. In addition, ibrutinib significantly improved locomotor functional recovery in all treated groups, especially in immediate and double-injection groups. Neural tissue protection and locomotor functional recovery suggest ibrutinib treatment as a potent immunotherapeutic intervention for traumatic SCI that warrants clinical testing.
Figure
Reference
-
References
1. Lim PA, Tow AM. 2007; Recovery and regeneration after spinal cord injury: a review and summary of recent literature. Ann Acad Med Singap. 36:49–57. PMID: 17285186.2. Abbaszadeh HA, Niknazar S, Darabi S, Ahmady Roozbahany N, Noori-Zadeh A, Ghoreishi SK, Khoramgah MS, Sadeghi Y. 2018; Stem cell transplantation and functional recovery after spinal cord injury: a systematic review and meta-analysis. Anat Cell Biol. 51:180–8. DOI: 10.5115/acb.2018.51.3.180. PMID: 30310710. PMCID: PMC6172584.
Article3. Cristante AF, Barros Filho TE, Marcon RM, Letaif OB, Rocha ID. 2012; Therapeutic approaches for spinal cord injury. Clinics (Sao Paulo). 67:1219–24. DOI: 10.6061/clinics/2012(10)16. PMID: 23070351. PMCID: PMC3460027.
Article4. Thuret S, Moon LD, Gage FH. 2006; Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 7:628–43. DOI: 10.1038/nrn1955. PMID: 16858391.
Article5. Kwiecien JM, Dabrowski W, Dąbrowska-Bouta B, Sulkowski G, Oakden W, Kwiecien-Delaney CJ, Yaron JR, Zhang L, Schutz L, Marzec-Kotarska B, Stanisz GJ, Karis JP, Struzynska L, Lucas AR. 2020; Prolonged inflammation leads to ongoing damage after spinal cord injury. PLoS One. 15:e0226584. DOI: 10.1371/journal.pone.0226584. PMID: 32191733. PMCID: PMC7081990.
Article6. Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. 2006; The cellular inflammatory response in human spinal cords after injury. Brain. 129(Pt 12):3249–69. DOI: 10.1093/brain/awl296. PMID: 17071951.
Article7. Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. 2002; Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 22:7526–35. DOI: 10.1523/JNEUROSCI.22-17-07526.2002. PMID: 12196576. PMCID: PMC2792199.
Article8. Singh PL, Agarwal N, Barrese JC, Heary RF. 2012; Current therapeutic strategies for inflammation following traumatic spinal cord injury. Neural Regen Res. 7:1812–21. DOI: 10.3969/j.issn.1673-5374.2012.23.008. PMID: 25624806. PMCID: PMC4302532.9. Zhou H, Hu P, Yan X, Zhang Y, Shi W. 2020; Ibrutinib in chronic lymphocytic leukemia: clinical applications, drug resistance, and prospects. Onco Targets Ther. 13:4877–92. DOI: 10.2147/OTT.S249586. PMID: 32581549. PMCID: PMC7266824.10. Campbell R, Chong G, Hawkes EA. 2018; Novel indications for Bruton's tyrosine kinase inhibitors, beyond hematological malignancies. J Clin Med. 7:62. DOI: 10.3390/jcm7040062. PMID: 29561760. PMCID: PMC5920436.
Article11. Tobinai K, Ogura M, Ishizawa K, Suzuki T, Munakata W, Uchida T, Aoki T, Morishita T, Ushijima Y, Takahara S. 2016; Safety and tolerability of ibrutinib monotherapy in Japanese patients with relapsed/refractory B cell malignancies. Int J Hematol. 103:86–94. DOI: 10.1007/s12185-015-1900-3. PMID: 26588924.
Article12. Krupa A, Fol M, Rahman M, Stokes KY, Florence JM, Leskov IL, Khoretonenko MV, Matthay MA, Liu KD, Calfee CS, Tvinnereim A, Rosenfield GR, Kurdowska AK. 2014; Silencing Bruton's tyrosine kinase in alveolar neutrophils protects mice from LPS/immune complex-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 307:L435–48. DOI: 10.1152/ajplung.00234.2013. PMID: 25085625. PMCID: PMC4166786.
Article13. Hamasy A, Wang Q, Blomberg KE, Mohammad DK, Yu L, Vihinen M, Berglöf A, Smith CI. 2017; Substitution scanning identifies a novel, catalytically active ibrutinib-resistant BTK cysteine 481 to threonine (C481T) variant. Leukemia. 31:177–85. DOI: 10.1038/leu.2016.153. PMID: 27282255. PMCID: PMC5220130.
Article14. da Cunha-Bang C, Niemann CU. 2018; Targeting Bruton's tyrosine kinase across B-cell malignancies. Drugs. 78:1653–63. DOI: 10.1007/s40265-018-1003-6. PMID: 30390220.
Article15. de Porto AP, Liu Z, de Beer R, Florquin S, de Boer OJ, Hendriks RW, van der Poll T, de Vos AF. 2019; Btk inhibitor ibrutinib reduces inflammatory myeloid cell responses in the lung during murine pneumococcal pneumonia. Mol Med. 25:3. DOI: 10.1186/s10020-018-0069-7. PMID: 30646846. PMCID: PMC6332549.
Article16. Block H, Zarbock A. 2012; The role of the tec kinase Bruton's tyrosine kinase (Btk) in leukocyte recruitment. Int Rev Immunol. 31:104–18. DOI: 10.3109/08830185.2012.668982. PMID: 22449072.
Article17. Palumbo T, Nakamura K, Lassman C, Kidani Y, Bensinger SJ, Busuttil R, Kupiec-Weglinski J, Zarrinpar A. 2017; Bruton tyrosine kinase inhibition attenuates liver damage in a mouse warm ischemia and reperfusion model. Transplantation. 101:322–31. DOI: 10.1097/TP.0000000000001552. PMID: 27820779. PMCID: PMC5263143.
Article18. Ito M, Shichita T, Okada M, Komine R, Noguchi Y, Yoshimura A, Morita R. 2015; Bruton's tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat Commun. 6:7360. DOI: 10.1038/ncomms8360. PMID: 26059659. PMCID: PMC4490404.
Article19. Liu X, Zhang J, Han W, Wang Y, Liu Y, Zhang Y, Zhou D, Xiang L. 2017; Inhibition of BTK protects lungs from trauma-hemorrhagic shock-induced injury in rats. Mol Med Rep. 16:192–200. DOI: 10.3892/mmr.2017.6553. PMID: 28487990. PMCID: PMC5482099.
Article20. Florence JM, Krupa A, Booshehri LM, Davis SA, Matthay MA, Kurdowska AK. 2018; Inhibiting Bruton's tyrosine kinase rescues mice from lethal influenza-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 315:L52–8. DOI: 10.1152/ajplung.00047.2018. PMID: 29516781. PMCID: PMC6087894.
Article21. Zeraatpisheh Z, Mirzaei E, Nami M, Alipour H, Ghasemian S, Azari H, Aligholi H. 2020; A new and simple method for spinal cord injury induction in mice. Basic Clin Neurosci. Forthcoming.22. Unal B, Kaplan S, Odaci E, Aslan H, Aksak S, Unal D, Altunkaynak BZ, Gundogdu C, Gokyar A. 2009; Neuroprotective effects of methylprednisolone and hypothermia after experimental spinal cord injury: a histopathological and stereological study. Eurasian J Med. 41:169–74. PMID: 25610097. PMCID: PMC4261273.23. O'Connell KE, Mikkola AM, Stepanek AM, Vernet A, Hall CD, Sun CC, Yildirim E, Staropoli JF, Lee JT, Brown DE. 2015; Practical murine hematopathology: a comparative review and implications for research. Comp Med. 65:96–113. PMID: 25926395. PMCID: PMC4408895.24. Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG. 2006; Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma. 23:635–59. DOI: 10.1089/neu.2006.23.635. PMID: 16689667.
Article25. Jakeman LB. Chen J, Xu XM, Xu ZC, Zhang JH, editors. 2012. Assessment of lesion and tissue sparing volumes following spinal cord injury. Animal Models of Acute Neurological Injuries II. Humana Press;New York: p. 417–42. DOI: 10.1007/978-1-61779-782-8_37.
Article26. Lee SM, Rosen S, Weinstein P, van Rooijen N, Noble-Haeusslein LJ. 2011; Prevention of both neutrophil and monocyte recruitment promotes recovery after spinal cord injury. J Neurotrauma. 28:1893–907. DOI: 10.1089/neu.2011.1860. PMID: 21657851. PMCID: PMC3172879.
Article27. Hawthorne AL, Popovich PG. 2011; Emerging concepts in myeloid cell biology after spinal cord injury. Neurotherapeutics. 8:252–61. DOI: 10.1007/s13311-011-0032-6. PMID: 21400005. PMCID: PMC3101835.
Article28. Hendriks RW, Yuvaraj S, Kil LP. 2014; Targeting Bruton's tyrosine kinase in B cell malignancies. Nat Rev Cancer. 14:219–32. DOI: 10.1038/nrc3702. PMID: 24658273.
Article29. Blez D, Blaize M, Soussain C, Boissonnas A, Meghraoui-Kheddar A, Menezes N, Portalier A, Combadière C, Leblond V, Ghez D, Fekkar A. 2020; Ibrutinib induces multiple functional defects in the neutrophil response against Aspergillus fumigatus. Haematologica. 105:478–89. DOI: 10.3324/haematol.2019.219220. PMID: 31171644. PMCID: PMC7012467.30. Facchinelli D, Marchesini G, Nadali G, Pagano L. 2018; Invasive fungal infections in patients with chronic lymphoproliferative disorders in the era of target drugs. Mediterr J Hematol Infect Dis. 10:e2018063. DOI: 10.4084/mjhid.2018.063. PMID: 30416695. PMCID: PMC6223569.
Article31. Rogers K. 2018; Ibrutinib and fungus: an invasive concern. Blood. 131:1882–4. DOI: 10.1182/blood-2018-02-832154. PMID: 29699994.
Article32. Weber ANR, Bittner Z, Liu X, Dang TM, Radsak MP, Brunner C. 2017; Bruton's tyrosine kinase: an emerging key player in innate immunity. Front Immunol. 8:1454. DOI: 10.3389/fimmu.2017.01454. PMID: 29167667. PMCID: PMC5682317.
Article33. Stadler N, Hasibeder A, Lopez PA, Teschner D, Desuki A, Kriege O, Weber ANR, Schulz C, Michel C, Heβ G, Radsak MP. 2017; The Bruton tyrosine kinase inhibitor ibrutinib abrogates triggering receptor on myeloid cells 1-mediated neutrophil activation. Haematologica. 102:e191–4. DOI: 10.3324/haematol.2016.152017. PMID: 28126969. PMCID: PMC5477622.
Article34. Miao EA, Rajan JV, Aderem A. 2011; Caspase-1-induced pyroptotic cell death. Immunol Rev. 243:206–14. DOI: 10.1111/j.1600-065X.2011.01044.x. PMID: 21884178. PMCID: PMC3609431.
Article35. Dinarello CA. 2018; Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 281:8–27. DOI: 10.1111/imr.12621. PMID: 29247995. PMCID: PMC5756628.
Article36. Das D, Hong J. 2020; Irreversible kinase inhibitors targeting cysteine residues and their applications in cancer therapy. Mini Rev Med Chem. 20:1732–53. DOI: 10.2174/1389557520666200513121524. PMID: 32400331.
Article37. Neirinckx V, Coste C, Franzen R, Gothot A, Rogister B, Wislet S. 2014; Neutrophil contribution to spinal cord injury and repair. J Neuroinflammation. 11:150. DOI: 10.1186/s12974-014-0150-2. PMID: 25163400. PMCID: PMC4174328.
Article38. Stirling DP, Liu S, Kubes P, Yong VW. 2009; Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J Neurosci. 29:753–64. DOI: 10.1523/JNEUROSCI.4918-08.2009. PMID: 19158301. PMCID: PMC6665178.
Article39. Treon SP, Castillo JJ, Skarbnik AP, Soumerai JD, Ghobrial IM, Guerrera ML, Meid K, Yang G. 2020; The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 135:1912–5. DOI: 10.1182/blood.2020006288. PMID: 32302379. PMCID: PMC7243149.
Article40. Nastoupil LJ, Lunning MA, Vose JM, Schreeder MT, Siddiqi T, Flowers CR, Cohen JB, Burger JA, Wierda WG, O'Brien S, Sportelli P, Miskin HP, Purdom MA, Weiss MS, Fowler NH. 2019; Tolerability and activity of ublituximab, umbralisib, and ibrutinib in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: a phase 1 dose escalation and expansion trial. Lancet Haematol. 6:e100–9. DOI: 10.1016/S2352-3026(18)30216-3. PMID: 30709431.
Article41. Choi SH, Sung CH, Heo DR, Jeong SY, Kang CN. 2020; Incidence of acute spinal cord injury and associated complications of methylprednisolone therapy: a national population-based study in South Korea. Spinal Cord. 58:232–7. DOI: 10.1038/s41393-019-0357-2. PMID: 31527724.
Article42. Cheung V, Hoshide R, Bansal V, Kasper E, Chen CC. 2015; Methylprednisolone in the management of spinal cord injuries: lessons from randomized, controlled trials. Surg Neurol Int. 6:142. DOI: 10.4103/2152-7806.163452. PMID: 26392918. PMCID: PMC4553662.
Article43. Chikuda H, Yasunaga H, Takeshita K, Horiguchi H, Kawaguchi H, Ohe K, Fushimi K, Tanaka S. 2014; Mortality and morbidity after high-dose methylprednisolone treatment in patients with acute cervical spinal cord injury: a propensity-matched analysis using a nationwide administrative database. Emerg Med J. 31:201–6. DOI: 10.1136/emermed-2012-202058. PMID: 23449889. PMCID: PMC3932981.
Article44. Suberviola B, González-Castro A, Llorca J, Ortiz-Melón F, Miñambres E. 2008; Early complications of high-dose methylprednisolone in acute spinal cord injury patients. Injury. 39:748–52. DOI: 10.1016/j.injury.2007.12.005. PMID: 18541241.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Animals models of spinal cord contusion injury
- Insulin-like Growth Factor-1 Receptor Dictates Beneficial Effects of Treadmill Training by Regulating Survival and Migration of Neural Stem Cell Grafts in the Injured Spinal Cord
- Alendronate Enhances Functional Recovery after Spinal Cord Injury
- The Significance of Evoked Potentials according to the Injury Severity of Spinal Cord Contusive Rat Model
- Spinal Cord Regeneration in Rat using Neural Stem Cell Differentiated from Human Telencephalon