Diabetes Metab J.
2021 Sep;45(5):739-752. 10.4093/dmj.2020.0137.
Magnetic Resonance-Based Assessments Better Capture Pathophysiologic Profiles and Progression in Nonalcoholic Fatty Liver Disease
- Affiliations
-
- 1Department of Radiology, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 2Department of Surgery, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 3Division of Gastroenterology and Hepatology, Department of Internal Medicine, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 4Department of Family Medicine, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 5Department of Internal Medicine, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 6Department of Pathology, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 7Department of Preventive Medicine, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 8Siemens Healthineers Ltd., Seoul, Korea
- KMID: 2520856
- DOI: http://doi.org/10.4093/dmj.2020.0137
Abstract
- Background
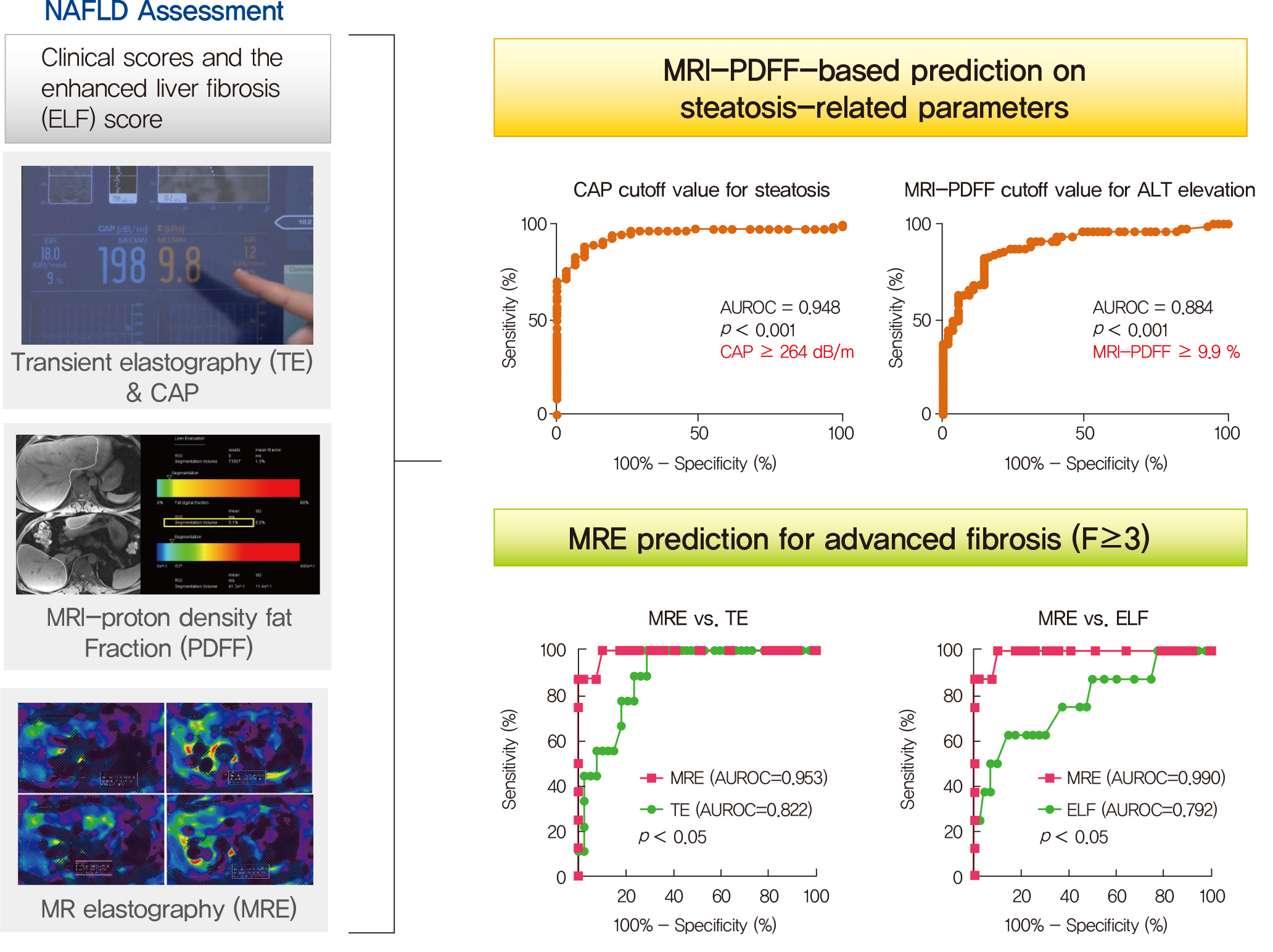
Several noninvasive tools are available for the assessment of nonalcoholic fatty liver disease (NAFLD) including clinical and blood biomarkers, transient elastography (TE), and magnetic resonance imaging (MRI) techniques, such as proton density fat fraction (MRI-PDFF) and magnetic resonance elastography (MRE). In the present study, we aimed to evaluate whether magnetic resonance (MR)-based examinations better discriminate the pathophysiologic features and fibrosis progression in NAFLD than other noninvasive methods.
Methods
A total of 133 subjects (31 healthy volunteers and 102 patients with NAFLD) were subjected to clinical and noninvasive NAFLD evaluation, with additional liver biopsy in some patients (n=54).
Results
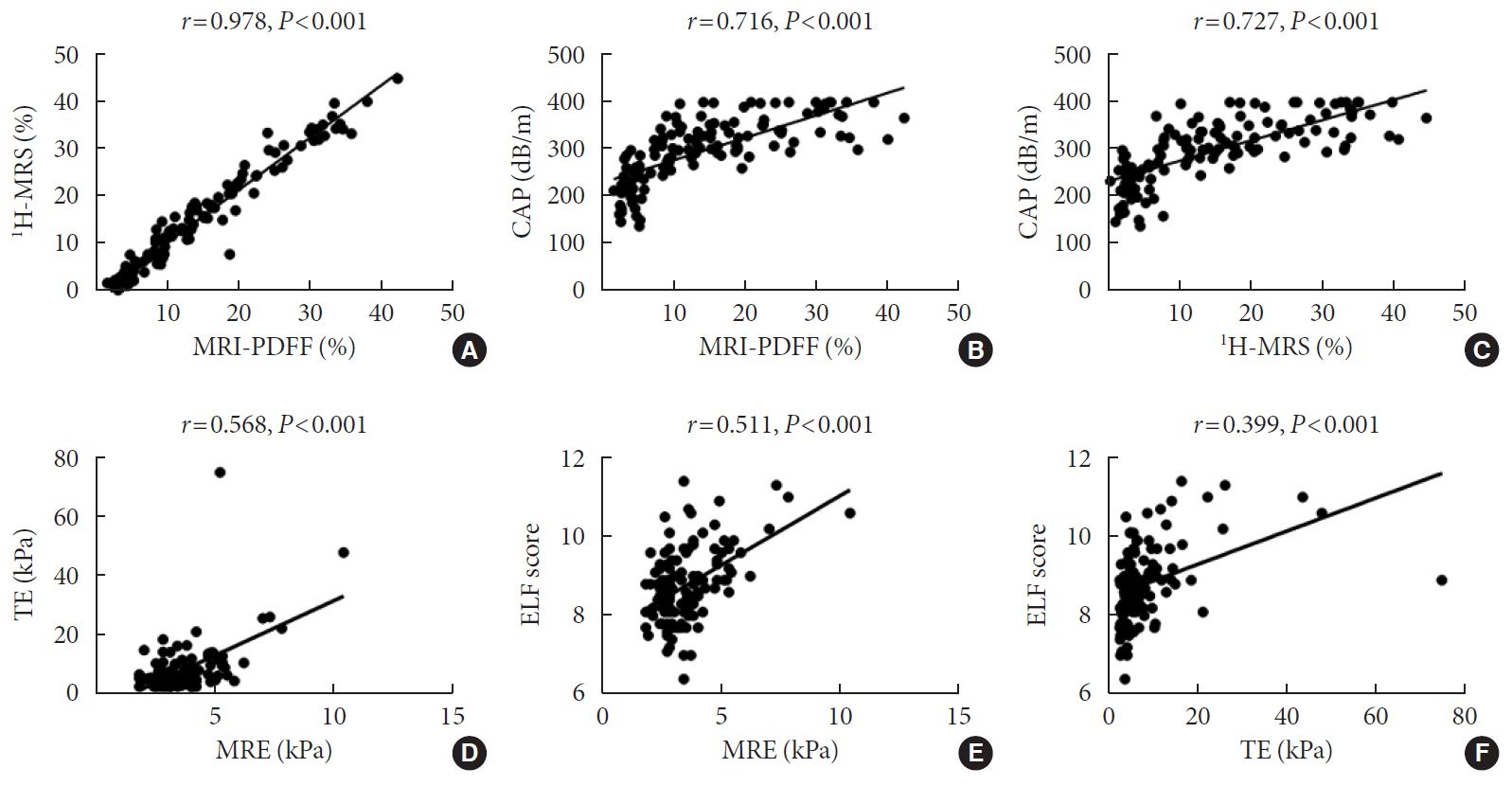
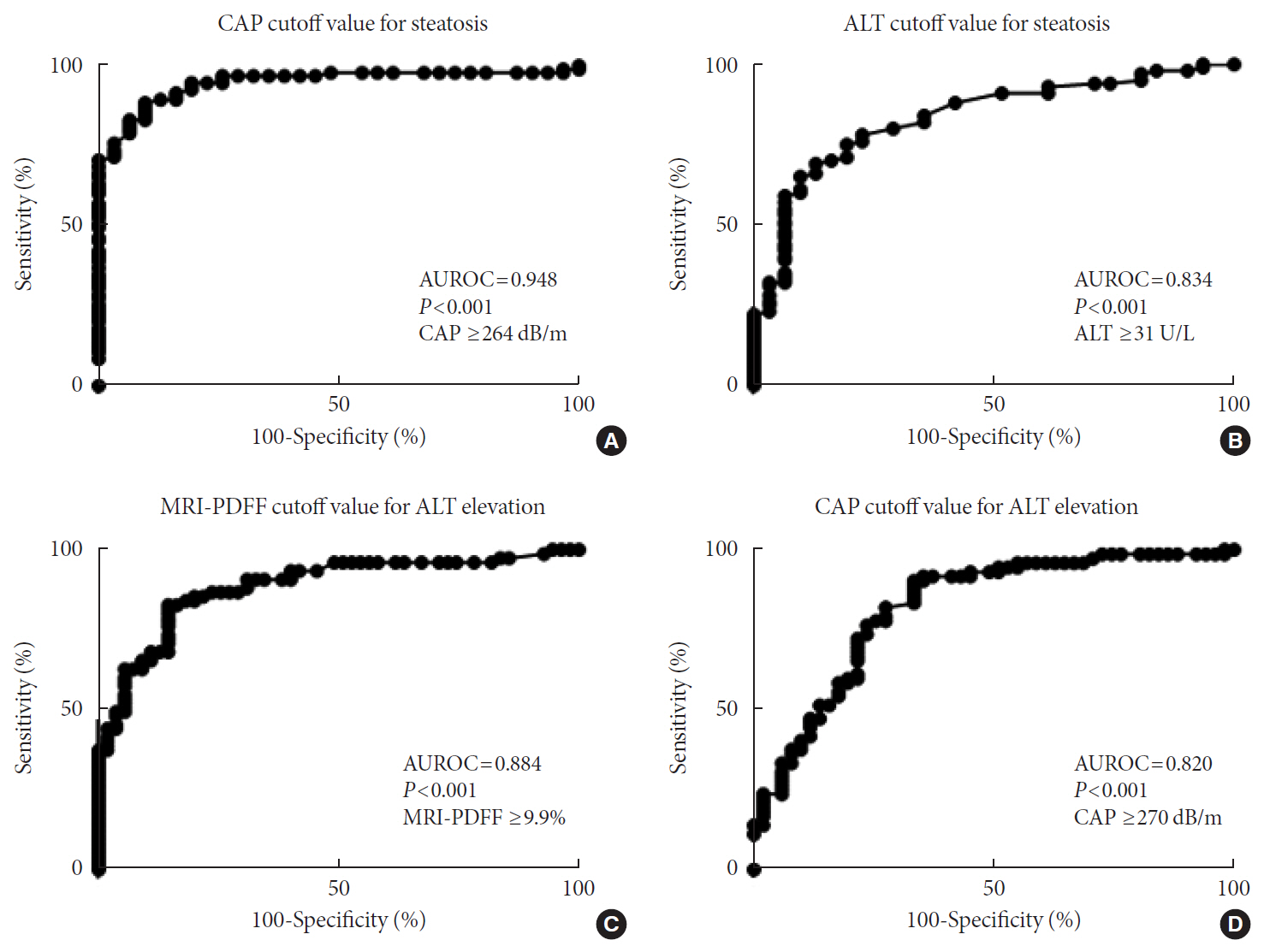
MRI-PDFF correlated far better with hepatic fat measured by MR spectroscopy (r=0.978, P<0.001) than with the TE controlled attenuation parameter (CAP) (r=0.727, P<0.001). In addition, MRI-PDFF showed stronger correlations with various pathophysiologic parameters for cellular injury, glucose and lipid metabolism, and inflammation, than the TE-CAP. The MRI-PDFF and TE-CAP cutoff levels associated with abnormal elevation of serum alanine aminotransferase were 9.9% and 270 dB/m, respectively. The MRE liver stiffness measurement (LSM) showed stronger correlations with liver enzymes, platelets, complement component 3, several clinical fibrosis scores, and the enhanced liver fibrosis (ELF) score than the TE-LSM. In an analysis of only biopsied patients, MRE performed better in discriminating advanced fibrosis with a cutoff value of 3.9 kPa than the TE (cutoff 8.1 kPa) and ELF test (cutoff 9.2 kPa).
Conclusion
Our results suggest that MRI-based assessment of NAFLD is the best non-invasive tool that captures the histologic, pathophysiologic and metabolic features of the disease.
Keyword
Figure
Cited by 1 articles
-
A Composite Blood Biomarker Including AKR1B10 and Cytokeratin 18 for Progressive Types of Nonalcoholic Fatty Liver Disease
Seung Joon Choi, Sungjin Yoon, Kyoung-Kon Kim, Doojin Kim, Hye Eun Lee, Kwang Gi Kim, Seung Kak Shin, Ie Byung Park, Seong Min Kim, Dae Ho Lee
Diabetes Metab J. 2024;48(4):740-751. doi: 10.4093/dmj.2023.0189.
Reference
-
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016; 64:73–84.
Article2. Lee YH, Cho Y, Lee BW, Park CY, Lee DH, Cha BS, et al. Nonalcoholic fatty liver disease in diabetes. Part I: epidemiology and diagnosis. Diabetes Metab J. 2019; 43:31–45.
Article3. Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab. 2015; 100:2231–8.
Article4. Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011; 43:617–49.
Article5. Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015; 61:1547–54.
Article6. Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015; 149:389–97.
Article7. Bannas P, Kramer H, Hernando D, Agni R, Cunningham AM, Mandal R, et al. Quantitative magnetic resonance imaging of hepatic steatosis: Validation in ex vivo human livers. Hepatology. 2015; 62:1444–55.8. Castera L. Noninvasive evaluation of nonalcoholic fatty liver disease. Semin Liver Dis. 2015; 35:291–303.
Article9. Wong VW, Vergniol J, Wong GL, Foucher J, Chan HL, Le Bail B, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010; 51:454–62.
Article10. de Ledinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014; 60:1026–31.11. Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol. 2016; 65:1006–16.
Article12. Shi Y, Xia F, Li QJ, Li JH, Yu B, Li Y, et al. Magnetic resonance elastography for the evaluation of liver fibrosis in chronic hepatitis B and C by using both gradient-recalled echo and spinecho echo planar imaging: a prospective study. Am J Gastroenterol. 2016; 111:823–33.
Article13. Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017; 152:598–607.
Article14. Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology. 2015; 61:1239–50.
Article15. Le TA, Chen J, Changchien C, Peterson MR, Kono Y, Patton H, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012; 56:922–32.
Article16. Jayakumar S, Middleton MS, Lawitz EJ, Mantry PS, Caldwell SH, Arnold H, et al. Longitudinal correlations between MRE, MRI-PDFF, and liver histology in patients with non-alcoholic steatohepatitis: analysis of data from a phase II trial of selonsertib. J Hepatol. 2019; 70:133–41.
Article17. Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008; 47:455–60.
Article18. Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008; 135:32–40.
Article19. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41:1313–21.
Article20. Caussy C, Alquiraish MH, Nguyen P, Hernandez C, Cepin S, Fortney LE, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. 2018; 67:1348–59.
Article21. Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013; 37:544–55.
Article22. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–45.
Article23. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018; 67:328–57.
Article24. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018; 67:1560–99.
Article25. Newsome PN, Cramb R, Davison SM, Dillon JF, Foulerton M, Godfrey EM, et al. Guidelines on the management of abnormal liver blood tests. Gut. 2018; 67:6–19.
Article26. Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, et al. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. 2018; 68:349–60.
Article27. Shen F, Zheng RD, Shi JP, Mi YQ, Chen GF, Hu X, et al. Impact of skin capsular distance on the performance of controlled attenuation parameter in patients with chronic liver disease. Liver Int. 2015; 35:2392–400.
Article28. Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Ledinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017; 66:1022–30.
Article29. Petta S, Wong VW, Camma C, Hiriart JB, Wong GL, Marra F, et al. Improved noninvasive prediction of liver fibrosis by liver stiffness measurement in patients with nonalcoholic fatty liver disease accounting for controlled attenuation parameter values. Hepatology. 2017; 65:1145–55.
Article30. Vuppalanchi R, Siddiqui MS, Van Natta ML, Hallinan E, Brandman D, Kowdley K, et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018; 67:134–44.
Article31. Kotronen A, Westerbacka J, Bergholm R, Pietilainen KH, YkiJarvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007; 92:3490–7.
Article32. Wildman-Tobriner B, Middleton MM, Moylan CA, Rossi S, Flores O, Chang ZA, et al. Association between magnetic resonance imaging-proton density fat fraction and liver histology features in patients with nonalcoholic fatty liver disease or nonalcoholic steatohepatitis. Gastroenterology. 2018; 155:1428–35.33. Yki-Jarvinen H. Diagnosis of non-alcoholic fatty liver disease (NAFLD). Diabetologia. 2016; 59:1104–11.
Article34. Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016; 150:626–37.
Article35. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019; 156:1264–81.
Article36. Vilar-Gomez E, Chalasani N. Non-invasive assessment of non-alcoholic fatty liver disease: clinical prediction rules and bloodbased biomarkers. J Hepatol. 2018; 68:305–15.
Article37. Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan(®)) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease: where do we stand? World J Gastroenterol. 2016; 22:7236–51.38. McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010; 59:1265–9.
Article39. Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013; 59:236–42.
Article40. Rensen SS, Slaats Y, Driessen A, Peutz-Kootstra CJ, Nijhuis J, Steffensen R, et al. Activation of the complement system in human nonalcoholic fatty liver disease. Hepatology. 2009; 50:1809–17.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Noninvasive serum biomarkers for liver steatosis in nonalcoholic fatty liver disease: Current and future developments
- The crosstalk between insulin resistance and nonalcoholic fatty liver disease/metabolic dysfunction-associated fatty liver disease: a culprit or a consequence?
- New Perspectives in Pediatric Nonalcoholic Fatty Liver Disease: Epidemiology, Genetics, Diagnosis, and Natural History
- Nonalcoholic fatty liver disease: pathogenesis and treatment
- Noninvasive Evaluation of Nonalcoholic Fatty Liver Disease