Nutr Res Pract.
2021 Oct;15(5):568-578. 10.4162/nrp.2021.15.5.568.
Psidium guajava L. leaf extract inhibits adipocyte differentiation and improves insulin sensitivity in 3T3-L1 cells
- Affiliations
-
- 1Department of Food Science and Nutrition, The Catholic University of Korea, Bucheon 14662, Korea
- KMID: 2520659
- DOI: http://doi.org/10.4162/nrp.2021.15.5.568
Abstract
- BACKGROUND/OBJECTIVES
Psidium guajava L. (guava) leaves have been shown to exhibit hypoglycemic and antidiabetic effects in rodents. This study investigated the effects of guava leaf extract on adipogenesis, glucose uptake, and lipolysis of adipocytes to examine whether the antidiabetic properties are mediated through direct effects on adipocytes.
MATERIALS/METHODS
3T3-L1 cells were treated with 25, 50, 100 µg/mL of methanol extract from guava leaf extract (GLE) or 0.1% dimethyl sulfoxide as a control. Lipid accumulation was evaluated with Oil Red O Staining and AdipoRed assay. Immunoblotting was performed to measure the expression of adipogenic transcription factors, fatty acid synthase (FAS), and AMP-activated protein kinase (AMPK). Glucose uptake under basal or insulin-stimulated condition was measured using a glucose analog 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose. Lipolysis from fully differentiated adipocytes was measured by free fatty acids release into the culture medium in the presence or absence of epinephrine.
RESULTS
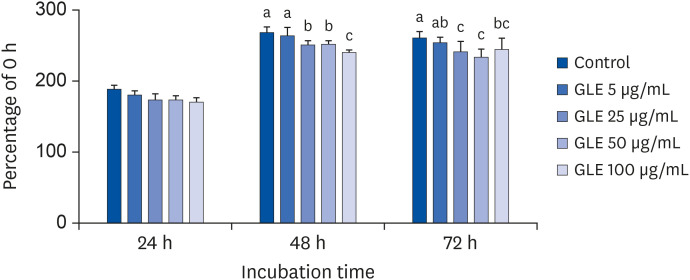
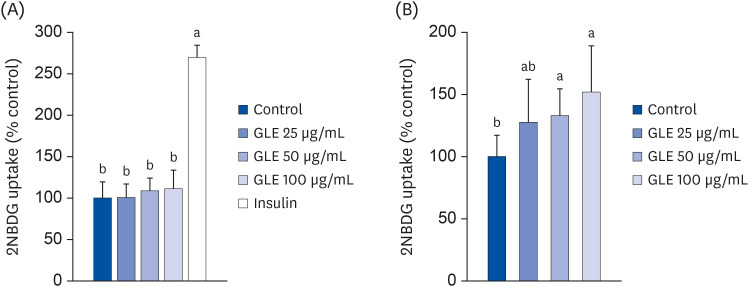
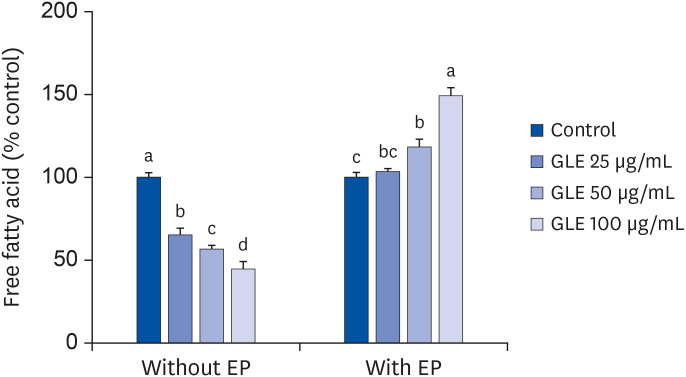
Oil Red O staining and AdipoRed assay have shown that GLE treatment reduced lipid accumulation during adipocyte differentiation. Mitotic clonal expansion, an early essential event for adipocyte differentiation, was inhibited by GLE treatment. GLE inhibited the expression of transcription factors involved in adipocyte differentiation, such as peroxisome proliferator-activated receptor γ (PPARγ), CCAAT/enhancer-binding protein α (C/EBPα), and sterol regulatory element-binding protein-1c (SREBP-1c). FAS expression was also decreased while the phosphorylation of AMPK was increased by GLE treatment. In addition, GLE increased insulin-induced glucose uptake into adipocytes. In lipid-filled mature adipocytes, GLE enhanced epinephrine-induced lipolysis but reduced basal lipolysis dose-dependently.
CONCLUSIONS
The results show that GLE inhibits adipogenesis and improves adipocyte function by reducing basal lipolysis and increasing insulin-stimulated glucose uptake in adipocytes, which can be partly associated with antidiabetic effects of guava leaves.
Figure
Reference
-
1. International Diabetes Federation. IDF Diabetes Atlas 9th Edition. Brussels, Belgium: International Diabetes Federation;2019. cited 2020 August 10. Available from: https://www.diabetesatlas.org/.2. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017; 389:2239–2251. PMID: 28190580.
Article3. Giaccari A, Sorice G, Muscogiuri G. Glucose toxicity: the leading actor in the pathogenesis and clinical history of type 2 diabetes - mechanisms and potentials for treatment. Nutr Metab Cardiovasc Dis. 2009; 19:365–377. PMID: 19428228.
Article4. Kalsi A, Singh S, Taneja N, Kukal S, Mani S. Current treatments for type 2 diabetes, their side effects and possible complementary treatments. Int J Pharm Pharm Sci. 2015; 7:13–18.5. Gutiérrez RM, Mitchell S, Solis RV. Psidium guajava: a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2008; 117:1–27. PMID: 18353572.6. Khan HB, Shanmugavalli R, Rajendran D, Bai MR, Sorimuthu S. Protective effect of Psidium guajava leaf extract on altered carbohydrate metabolism in streptozotocin-induced diabetic rats. J Diet Suppl. 2013; 10:335–344. PMID: 24237189.7. Ojewole JA. Hypoglycemic and hypotensive effects of Psidium guajava Linn. (Myrtaceae) leaf aqueous extract. Methods Find Exp Clin Pharmacol. 2005; 27:689–695. PMID: 16395418.8. Deguchi Y, Miyazaki K. Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutr Metab (Lond). 2010; 7:9. PMID: 20181067.
Article9. Wang B, Liu HC, Hong JR, Li HG, Huang CY. Effect of Psidium guajava leaf extract on alpha-glucosidase activity in small intestine of diabetic mouse. Sichuan Da Xue Xue Bao Yi Xue Ban. 2007; 38:298–301. PMID: 17441354.10. Shen SC, Cheng FC, Wu NJ. Effect of guava (Psidium guajava Linn.) leaf soluble solids on glucose metabolism in type 2 diabetic rats. Phytother Res. 2008; 22:1458–1464. PMID: 18819164.11. Vinayagam R, Jayachandran M, Chung SS, Xu B. Guava leaf inhibits hepatic gluconeogenesis and increases glycogen synthesis via AMPK/ACC signaling pathways in streptozotocin-induced diabetic rats. Biomed Pharmacother. 2018; 103:1012–1017. PMID: 29710658.
Article12. Beidokhti MN, Eid HM, Villavicencio MLS, Jäger AK, Lobbens ES, Rasoanaivo PR, McNair LM, Haddad PS, Staerk D. Evaluation of the antidiabetic potential of Psidium guajava L. (Myrtaceae) using assays for α-glucosidase, α-amylase, muscle glucose uptake, liver glucose production, and triglyceride accumulation in adipocytes. J Ethnopharmacol. 2020; 257:112877. PMID: 32305639.13. Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001; 409:729–733. PMID: 11217863.
Article14. Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006; 17:4–12. PMID: 16613757.15. McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002; 51:7–18. PMID: 11756317.
Article16. Ali AT, Hochfeld WE, Myburgh R, Pepper MS. Adipocyte and adipogenesis. Eur J Cell Biol. 2013; 92:229–236. PMID: 23876739.
Article17. Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996; 87:377–389. PMID: 8898192.
Article18. Moseti D, Regassa A, Kim WK. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int J Mol Sci. 2016; 17:124.
Article19. Guo X, Yoshitomi H, Gao M, Qin L, Duan Y, Sun W, Xu T, Xie P, Zhou J, Huang L, Liu T. Guava leaf extracts promote glucose metabolism in SHRSP.Z-Leprfa/Izm rats by improving insulin resistance in skeletal muscle. BMC Complement Altern Med. 2013; 13:52. PMID: 23452929.
Article20. Yang Q, Wen YM, Shen J, Chen MM, Wen JH, Li ZM, Liang YZ, Xia N. Guava leaf extract attenuates insulin resistance via the PI3K/Akt signaling pathway in a type 2 diabetic mouse model. Diabetes Metab Syndr Obes. 2020; 13:713–718. PMID: 32214834.21. Sim SY, Shin YE, Kim HK. Fucoidan from Undaria pinnatifida has anti-diabetic effects by stimulation of glucose uptake and reduction of basal lipolysis in 3T3-L1 adipocytes. Nutr Res. 2019; 65:54–62. PMID: 30952503.22. Kim JN, Han SN, Kim HK. Phytic acid and myo-inositol support adipocyte differentiation and improve insulin sensitivity in 3T3-L1 cells. Nutr Res. 2014; 34:723–731. PMID: 25174657.
Article23. Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci U S A. 2003; 100:44–49. PMID: 12502791.
Article24. Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006; 4:263–273. PMID: 17011499.
Article25. Chang E, Kim CY. Natural products and obesity: a focus on the regulation of mitotic clonal expansion during adipogenesis. Molecules. 2019; 24:1157.
Article26. Ceddia RB. The role of AMP-activated protein kinase in regulating white adipose tissue metabolism. Mol Cell Endocrinol. 2013; 366:194–203. PMID: 22750051.
Article27. Kola B, Grossman AB, Korbonits M. The role of AMP-activated protein kinase in obesity. Front Horm Res. 2008; 36:198–211. PMID: 18230904.
Article28. Daval M, Foufelle F, Ferré P. Functions of AMP-activated protein kinase in adipose tissue. J Physiol. 2006; 574:55–62. PMID: 16709632.
Article29. Zhou Y, Wang D, Zhu Q, Gao X, Yang S, Xu A, Wu D. Inhibitory effects of A-769662, a novel activator of AMP-activated protein kinase, on 3T3-L1 adipogenesis. Biol Pharm Bull. 2009; 32:993–998. PMID: 19483304.
Article30. Habinowski SA, Witters LA. The effects of AICAR on adipocyte differentiation of 3T3-L1 cells. Biochem Biophys Res Commun. 2001; 286:852–856. PMID: 11527376.
Article31. Langin D, Dicker A, Tavernier G, Hoffstedt J, Mairal A, Rydén M, Arner E, Sicard A, Jenkins CM, Viguerie N, van Harmelen V, Gross RW, Holm C, Arner P. Adipocyte lipases and defect of lipolysis in human obesity. Diabetes. 2005; 54:3190–3197. PMID: 16249444.
Article32. Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009; 48:275–297. PMID: 19464318.
Article33. Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie. 2016; 125:259–266. PMID: 26542285.
Article34. Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994; 93:2438–2446. PMID: 8200979.
Article35. Lam TK, Carpentier A, Lewis GF, van de Werve G, Fantus IG, Giacca A. Mechanisms of the free fatty acid-induced increase in hepatic glucose production. Am J Physiol Endocrinol Metab. 2003; 284:E863–73. PMID: 12676648.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- Effects of (6)-gingerol, ginger component on adipocyte development and differentiation in 3T3-L1
- TonEBP suppresses adipocyte differentiation via modulation of early signaling in 3T3-L1 cells
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3